SECOND SESSION
Dr. Herbert F. Mitchell presents
WONDERS OF GOD'S CREATION
The Universe, the Earth, and Life on Earth
Theme: “In the beginning, God created the heavens and the earth.” Genesis 1:1.
Life on Earth
In the First Session we looked at the universe around us ― the stars and their galaxies, the Sun and its planets, moons,
asteroids, comets, and meteors ― the “Big Bang” theory of how it all came about, and what the Bible has to say about these
events. We also examined the formation and early life of the Earth and Moon, and saw how the very first form of life ―
bacteria ― undoubtedly provided the oxygen we now have in our atmosphere, which not only provides us the breath of life,
but also through the special form of oxygen ― ozone ― protects us from the ultraviolet rays of the sun and other
stars. In this Session we are going to study the basis of all life ― the biological cell.
The Biological Cell
Before studying the biological cell, in which a lot of organic chemistry takes place, you might want to review some basic concepts in
that discipline.
Click here to review concepts of organic chemistry.
In studying life, the logical starting place is the cell, since the cell is the fundamental unit of all living things. Before the
microscope permitted the details of cells to be examined, life scientists thought them to be quite simple blobs of protoplasm. How
wrong they were!
As in the First Session we will present major topics at two levels ― relatively simple ― and more detailed.
Click here for next page or Click here for the more detailed examination of the contents of an animal
cell.
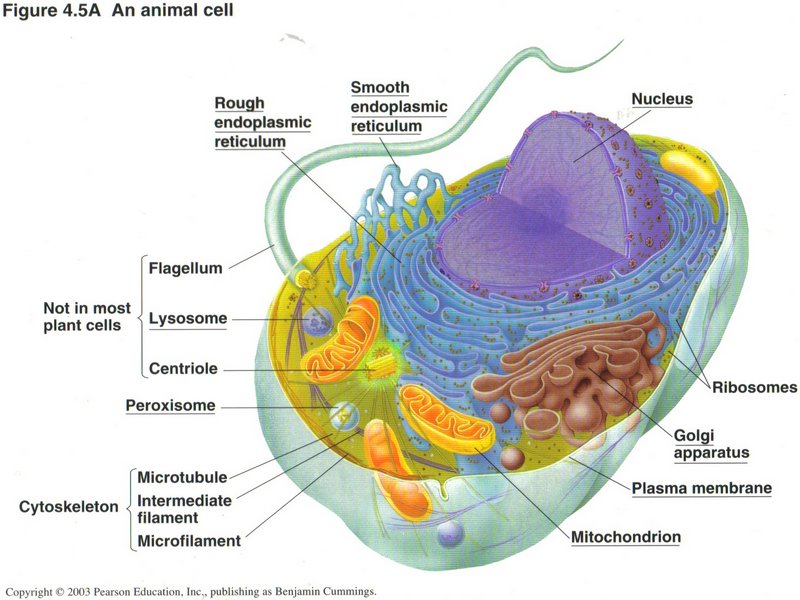 Here we see the structure of the eukaryotic (true nucleus) type of cell found in all life forms except bacteria; it is obviously
extremely complex. In this typical animal cell, the nucleus dominates the cell and contains the principal part of the DNA. It is in
the nucleus that the DNA is duplicated, a process called replication. It is
also in the nucleus that the generation of RNAs takes place in the process called transcription. There are several forms of
these. Messenger RNA (mRNA) is the ribbon from which proteins will be made in the ribosomes ― those little red dots
peppering the surfaces of the nuclear envelope and the rough ER (rough endoplasmic reticulum) ― in the process called
translation. Ribosomes are formed from another type of RNA ― rRNA (ribosonal RNA) ― plus several complex proteins. A
third type of RNA ― tRNA (transfer RNA) finds the specific amino acids needed to compose the protein and brings them to the
ribosome. The rough ER finishes the processing of the proto-protein (called a polypeptide) by chemically altering some of its
amino acid constituents and usually by folding it into its final shape. The rough ER also manufactures a number of chemical molecules
needed by the cell, like membrane units and enclosed vehicles, called vesicles, for carrying specific substances. The smooth
ER (smooth endoplasmic reticulum) manufactures fats and carbohydrates, including the sugars, needed to synthesize DNA and RNA.
Here we see the structure of the eukaryotic (true nucleus) type of cell found in all life forms except bacteria; it is obviously
extremely complex. In this typical animal cell, the nucleus dominates the cell and contains the principal part of the DNA. It is in
the nucleus that the DNA is duplicated, a process called replication. It is
also in the nucleus that the generation of RNAs takes place in the process called transcription. There are several forms of
these. Messenger RNA (mRNA) is the ribbon from which proteins will be made in the ribosomes ― those little red dots
peppering the surfaces of the nuclear envelope and the rough ER (rough endoplasmic reticulum) ― in the process called
translation. Ribosomes are formed from another type of RNA ― rRNA (ribosonal RNA) ― plus several complex proteins. A
third type of RNA ― tRNA (transfer RNA) finds the specific amino acids needed to compose the protein and brings them to the
ribosome. The rough ER finishes the processing of the proto-protein (called a polypeptide) by chemically altering some of its
amino acid constituents and usually by folding it into its final shape. The rough ER also manufactures a number of chemical molecules
needed by the cell, like membrane units and enclosed vehicles, called vesicles, for carrying specific substances. The smooth
ER (smooth endoplasmic reticulum) manufactures fats and carbohydrates, including the sugars, needed to synthesize DNA and RNA.
Click here for next page
 The Golgi apparatus is the clearing house for cell traffic vesicles of all kinds. It also manufactures vesicles and
lysosomes, which are membrane enclosed vesicles containing potent enzymes capable of breaking down most complex organic
molecules into simpler substances used in manufacture in the smooth and rough ERs.
The Golgi apparatus is the clearing house for cell traffic vesicles of all kinds. It also manufactures vesicles and
lysosomes, which are membrane enclosed vesicles containing potent enzymes capable of breaking down most complex organic
molecules into simpler substances used in manufacture in the smooth and rough ERs.
The cytoskeleton provides the supporting structures within the cell, both to separate the organelles and to provide pathways
by which various vesicles, RNAs, and other cellular entities are moved from one place to another within the cell. As you can see,
there are three types of these structural elements, each composed of small proteins which are continuously building up and breaking
down the rod-like elements shown.
The mitochondria are the powerhouses of the eukaryotic cell. They contain the machinery which transforms the energy of food
products (fats, sugars and starches) into the energy of the ATP (adenosine triphosphate) molecule, which is the universal
source of energy for all cells, as well as a component of DNA and RNA. There are two processes that perform this transformation:
glycolysis and the citric acid cycle plus chemiosmosis. Glycolysis, performed in both bacteria and higher
forms of life, generate two ATP from the basic food unit (glucose). Cells containing mitochondria generate as much as 38 ATP per
molecule of glucose, a tremendous increase in available energy. A mitochondrion has its own DNA, with all the genes and
protein-producing machinery found in the nucleus, which leads some biologists to believe it may have arisen from a bacterium.
This seems to me highly doubtful, since bacteria do not contain mitochondria, and mitochondria are dependent upon several vital
proteins produced only from the nucleus, in order to replicate.
Click here for next page
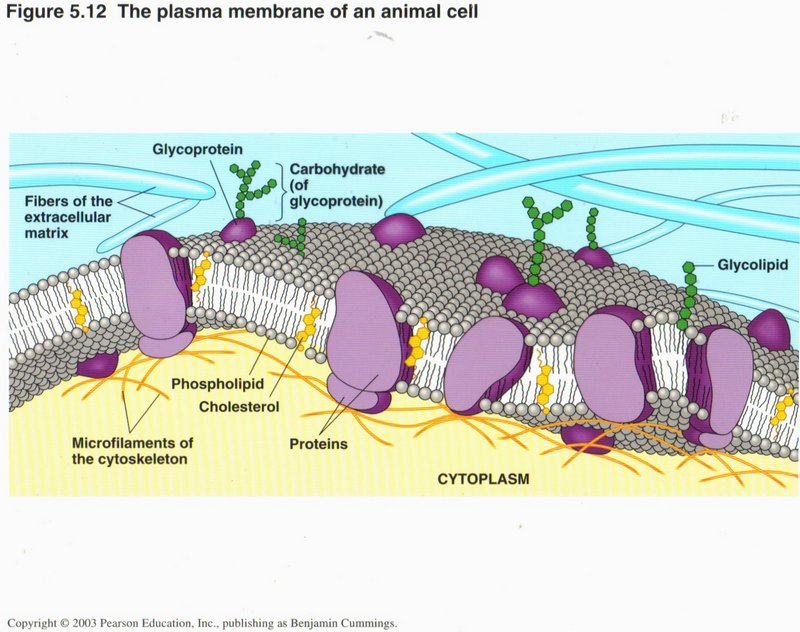 Here you can see something of the complexity of the plasma membrane surrounding the cell. Its main constituents are molecules of
phospholipids, which make up the double layer of the membrane. Since the fatty extensions (string-like molecules that point
to the center of the membrane) are water-repellant (like oil), the two layers of the membrane are continuously pressed together by
the watery media on the two sides (extra-cellular matrix on the outside and the cytoplasm on the inside). Studded throughout the
membrane are proteins of many kinds. Some provide recognition sites on the external side of the membrane for specific substances in
the extracellular medium. Some bind specialized messenger proteins (called hormones) that trigger many different functions in
the cell’s life cycle. Some provide pores which permit selected substances to enter or leave the cell. And some are involved with
moving ions (H+, Na+, Ca2+) (hydrogen, sodium, calcium positive ions) into or out of the cell.
Here you can see something of the complexity of the plasma membrane surrounding the cell. Its main constituents are molecules of
phospholipids, which make up the double layer of the membrane. Since the fatty extensions (string-like molecules that point
to the center of the membrane) are water-repellant (like oil), the two layers of the membrane are continuously pressed together by
the watery media on the two sides (extra-cellular matrix on the outside and the cytoplasm on the inside). Studded throughout the
membrane are proteins of many kinds. Some provide recognition sites on the external side of the membrane for specific substances in
the extracellular medium. Some bind specialized messenger proteins (called hormones) that trigger many different functions in
the cell’s life cycle. Some provide pores which permit selected substances to enter or leave the cell. And some are involved with
moving ions (H+, Na+, Ca2+) (hydrogen, sodium, calcium positive ions) into or out of the cell.
Click here for next page

Here you see a typical plant cell. As you can see, many of its elements are the same as in an animal cell. The principal exceptions
are the cell wall (usually of cellulose, and comprises the rigid structure of the plant), the central vacuole, which is
both a storage area for many cell products and a place where complex substances are broken down as in animal lysosomes, and the
chloroplast, the specialized organelle that converts the energy of sunlight into sugars and fats through photosynthesis.
Click here for next page
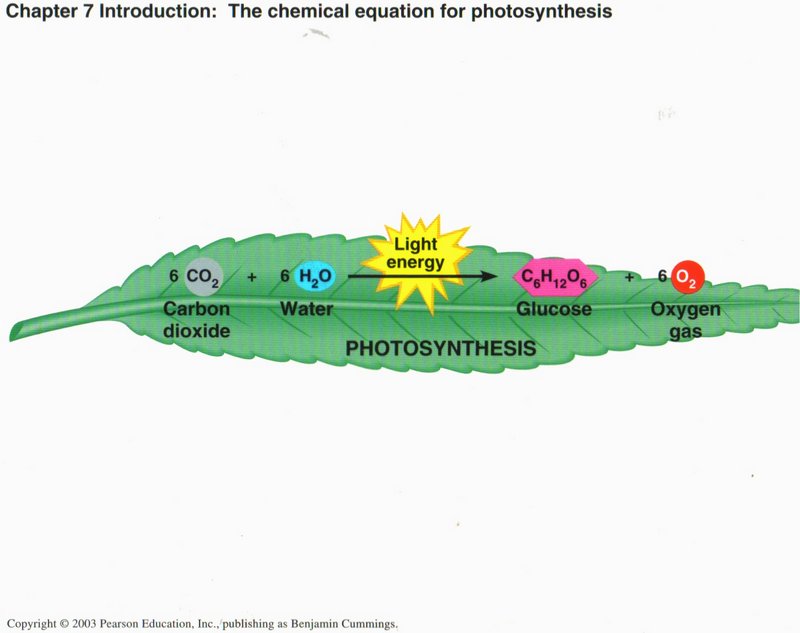 An Overview of Photosynthesis. Photosynthesis is the process by which autotrophic (self-feeding) plants and some other organisms
use light energy to make sugar and oxygen gas from carbon dioxide and water. The following chemical equation shows the net input and
output of photosynthesis:
An Overview of Photosynthesis. Photosynthesis is the process by which autotrophic (self-feeding) plants and some other organisms
use light energy to make sugar and oxygen gas from carbon dioxide and water. The following chemical equation shows the net input and
output of photosynthesis:
6 CO2 + 6 H2O + light energy —> C6H12O6 + 6 O2
Plants, some protists (algae), and some bacteria are photosynthetic autotrophs, the ultimate producers of food consumed by virtually
all living things.
Click here for next page
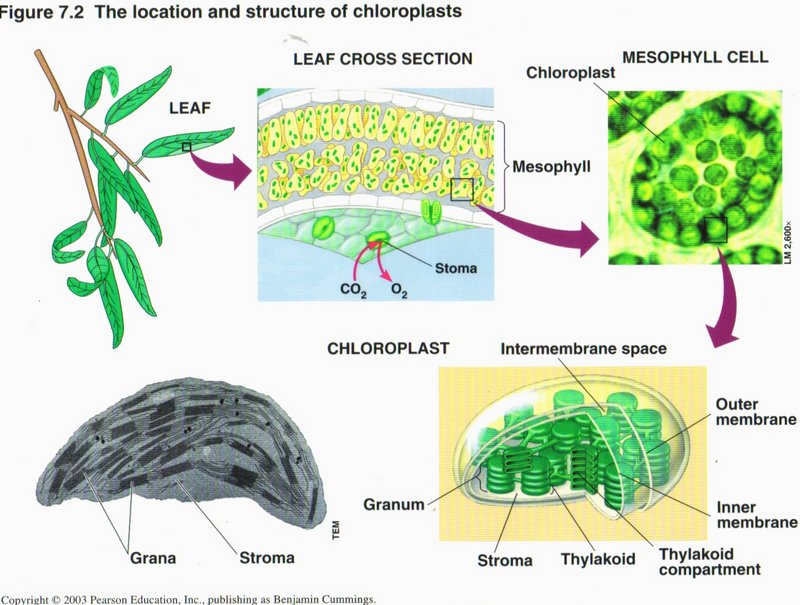
Photosynthesis
In most plants, photosynthesis occurs primarily in the leaves, in chloroplasts. A chloroplast contains stroma (a
fluid) and grana (stacks of thykaloids). The thykaloid membranes contain chlorophyll, the green pigment that captures light for
photosysthesis.
The complete process of photosynthesis consists of two linked sets of reactions: the light reactions and the Calvin cycle. The light reactions convert light energy to chemical
energy and produce O2. The Calvin cycle assembles sugar molecules from CO2 using the energy-carrying products of
the light reactions.
Click here for next page
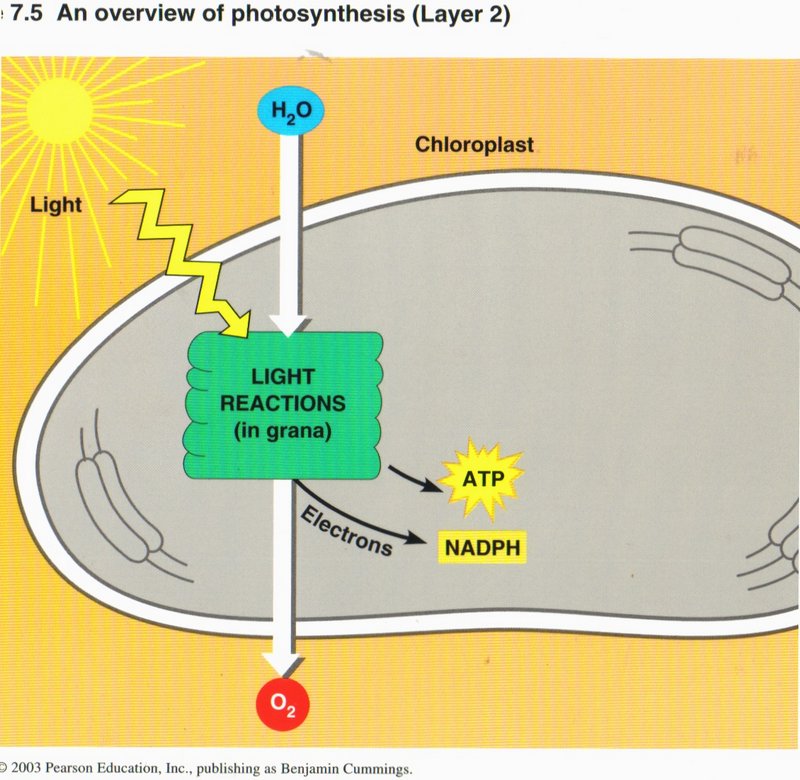
The Light Reactions: Converting Solar Energy to Chemical Energy. Certain wavelengths of visible light drive the light reactions
of photosynthesis (see left diagram). These light reactions break down the water (H2O) molecules and form (oxygen O2), NADPH and
ATP (energy carriers).
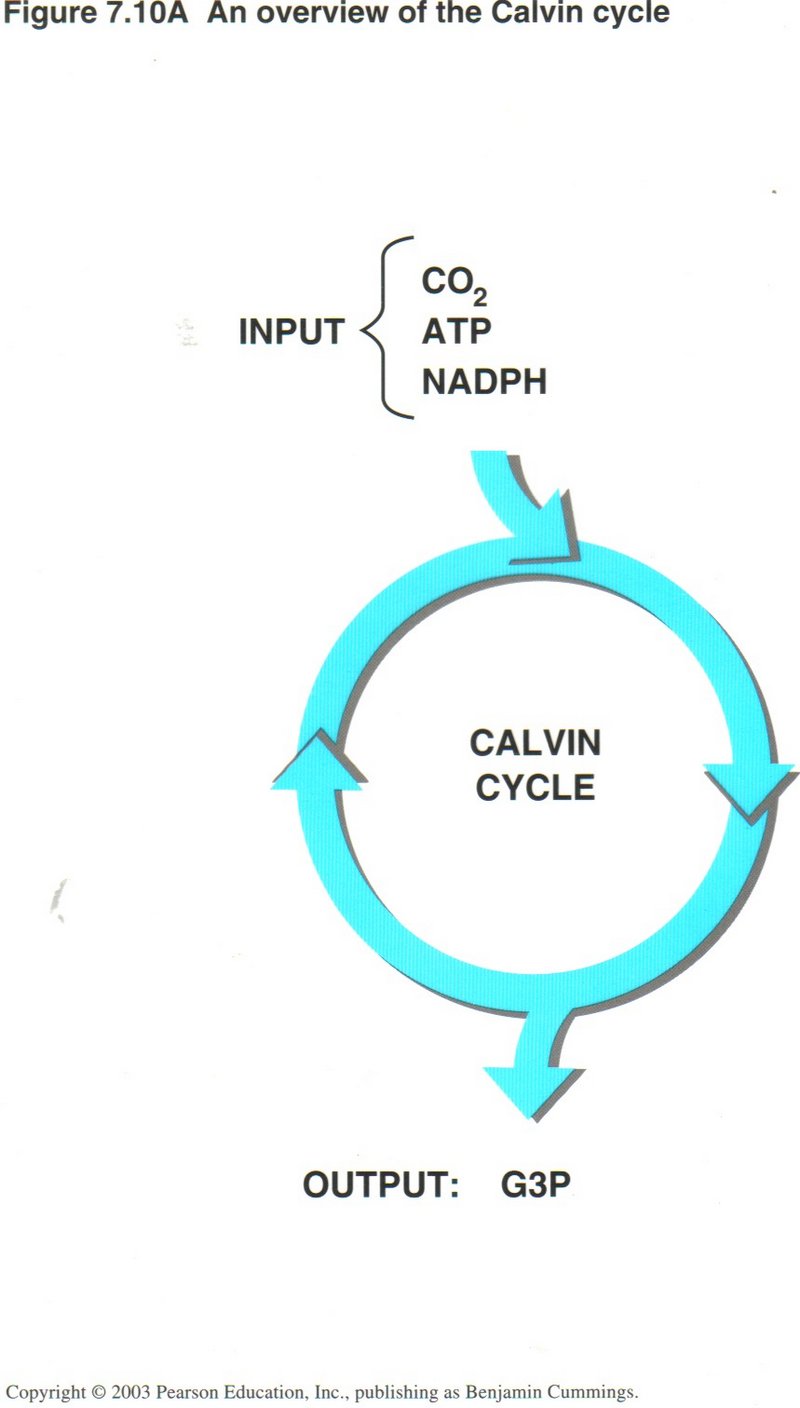
The Calvin Cycle: Converting CO2 to Sugars. The NADPH and ATP produced in the light reactions are used in the Calvin
cycle, which occurs in the chloroplast’s stroma (see right diagram). Using carbon from atmospheric CO2, and energy from ATPand NADPH, enzymes of the Calvin cycle
construct G3P, an energy-rich sugar. In turn, G3P is used to build glucose and other organic molecules.
Click here for next page
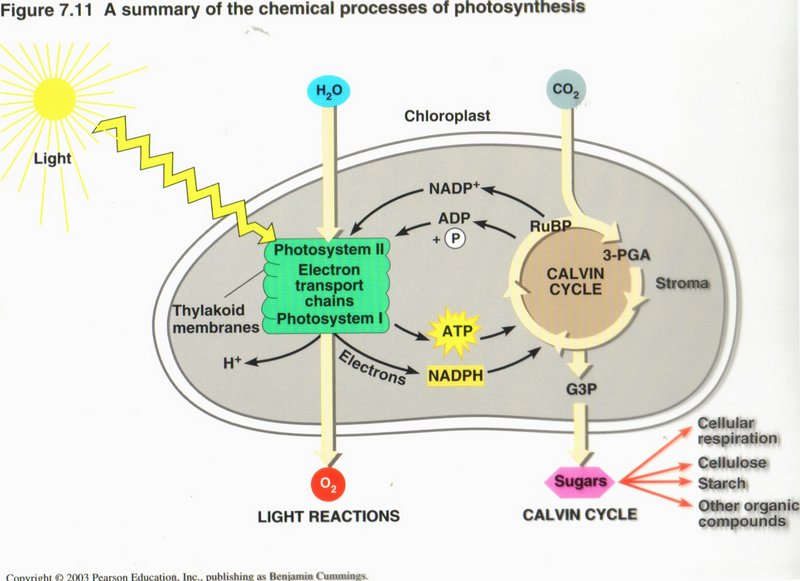
This figure summarizes photosynthesis. Many plants make more sugar than they need; the excess is stored in roots, tubers,and fruits,
and is a major food source for animals. Photosynthesis produces billions of tons of organic matter each year and thus sustains almost
all life on Earth. In most plants, stomata in the leaf surface usually close when the weather is hot and dry, shutting down
photosynthesis. Corn and sugarcane have a means of continuing photosynthesis, while pineapples, many cacti, and most succulents open
their stomata at night and make a four-carbon compound that is used as a CO2 source by the same cell during the day.
Click here for additional details of photosynthesis.
Photosynthesis, Solar Radiation, and Earth’s Atmosphere. Because of the increased burning of fossil fuels, atmospheric
CO2 is increasing. CO2 warms Earth’s surface by trapping heat in the atmosphere; this is called the greenhouse
effect. An excess of CO2 may cause the planet to overheat. Because photosynthesis removes CO2 from the
atmosphere, it could moderate the greenhouse effect; unfortunately, deforestation may cause a decline in the global rate of
photosynthesis. The O2 in the atmosphere results from photosynthesis. Solar radiation converts O2 high in the
atmosphere to ozone (O3), which shields organisms on Earth’s surface from the damaging effects of UV radiation. Industrial
chemicals called CFCs have hastened ozone breakdown, causing dangerous thinning of the ozone layer, but international restrictions on
these chemicals are allowing recovery.
Click here for next page.
DNA, the Blueprint of Life
Without doubt, DNA is the most important substance in the cell. British biologists Crick and Watson won the race for the first correct
description of the DNA molecule in 1953, for which they later won the Nobel prize. Since then, DNA has been a major field of study in
molecular biology. We have found out that DNA is the “blueprint of life.” It contains instructions for the generation of more than
40,000 different kinds of proteins and the many kinds of RNA, specialized areas called promoters or enhancers that can be recognized
by the appropriate proteins and thus determine where in the vast molecule transcription should begin, and finally vast lengths of
apparently useless base-pairs. Since it is the blue-print of life, it must somehow provide for the organization of the organism, and
for animals their instincts, to say nothing of the innate talents human beings possess at birth. DNA is subject to two processes,
described below: replication (duplication) and transcription (the first step in generating a protein). Both processes are 99.999%
accurate, but the few errors that do occur are responsible for many diseases and babies born with defects (as many as 1 out 600). So
you need to know something about DNA.
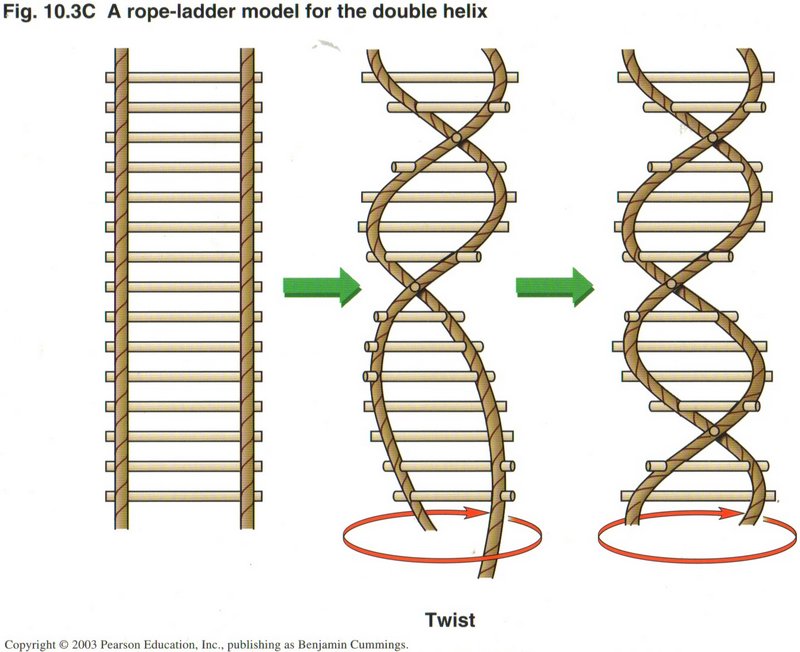
DNA is a helix, much like a twisted ladder. The twist may be gradual, as in the middle picture, or more twisted, as in the far right
picture. DNA is found in bacteria, plants, animals, and all other kinds of life. In all life forms except bacteria, the cells have
nuclei in which the vast majority of the DNA resides as extremely long linear molecules. In bacteria, the DNA ribbon is circular and
has roughly from 100,000 to 500,000 base-pairs. But plants also have circular DNA in their chloroplasts and all life forms except
bacteria have DNA in their mitochondria, whose molecule is also circular. No matter where the DNA is found, its instructions for
generating proteins and NRA use the same code, with very few exceptions.
Click here for next page
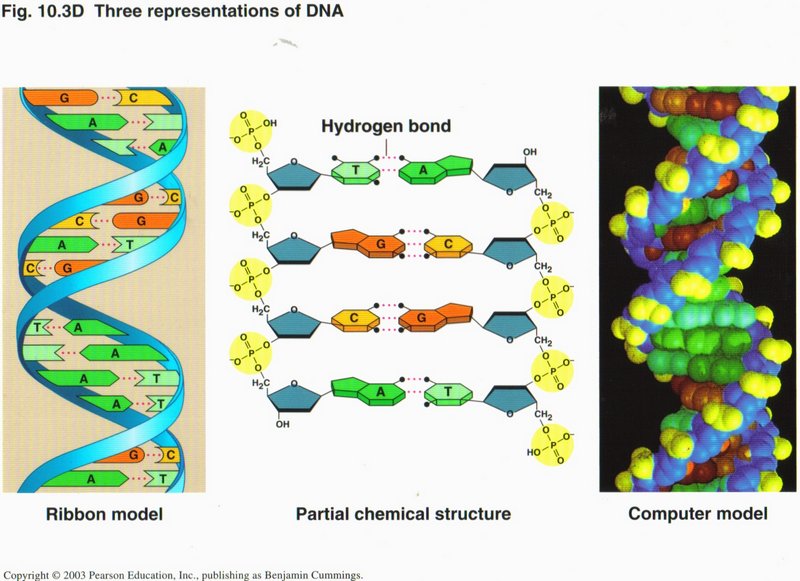
This view shows the helix pictorially at the left, in chemical symbol form in the center, and in computer simulation at the right.
In each representation, the same color code is used. The major elements are the sugar (blue) and phosphate structure (yellow), which
comprise the “legs” of the ladder, and the two bases (two shades each of green and orange) comprising the “rungs” of the ladder. The
atoms in each strand are connected by solid lines, representing covalence bonds, which are very strong and take energy to make or
break. The two strands are linked together by hydrogen bonds (dotted lines), which are only 1/100th as strong as covalence bonds, but
nevertheless hold the two strands together in the DNA helix.
Click here for next page
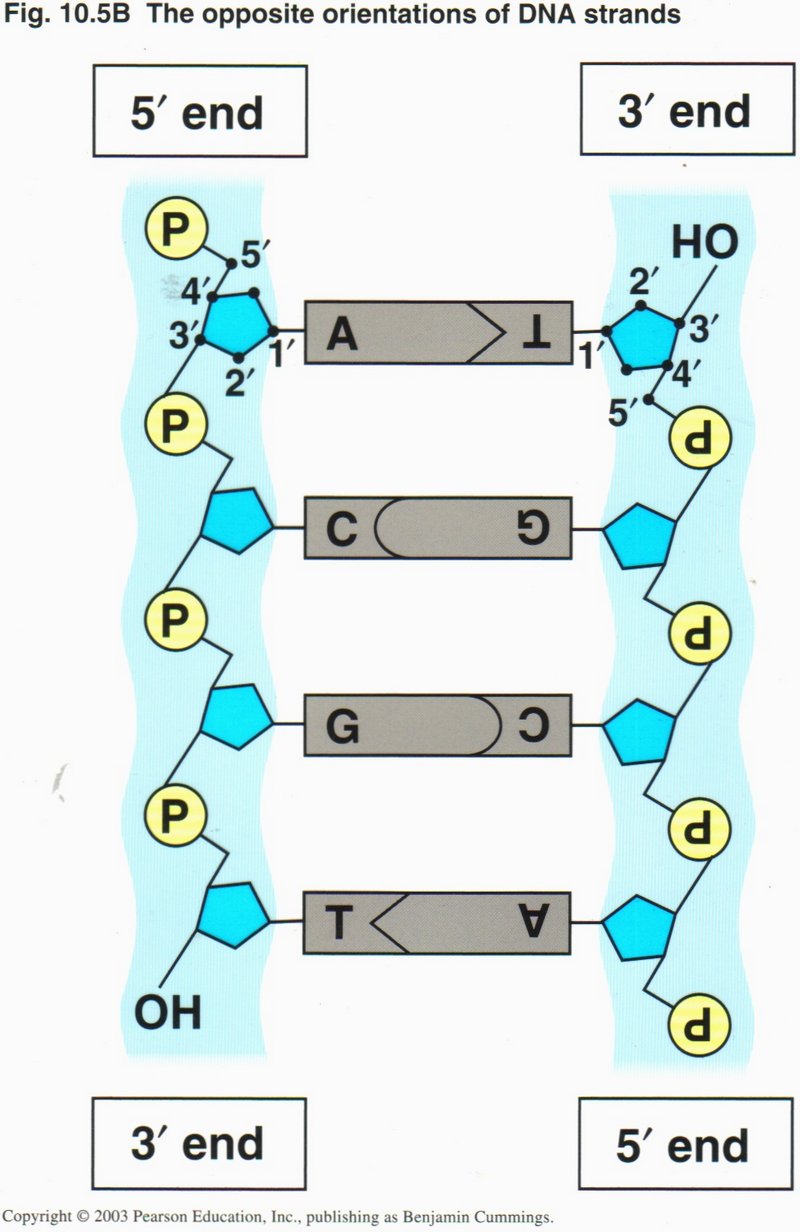
An enlargement of the previous representation shows that the two strands run in opposite directions in the helix, designated as the
5’ to 3’ directions ― so called from the numbers assigned to the carbon atoms attached the the phosphate complex (P) ― and so
constituted that the opposing central bases are always matched: "A" (adenine) with "T" (thymine) and "C" (cysteine) with
"G" (guanine). This particular pairing creates hydrogen bonds between the paired bases that are much stronger than other possible
pairings, and thus guarantees that the information contained in the helix is correct. The unit of DNA is the base-pair (also
called nucleotide). Although the nucleotides are shown as parallel to the phospho-sugar ladder, they are actually nearly at right
angles to the ladder, making the helix truly three-dimensional.
Click here for next page.
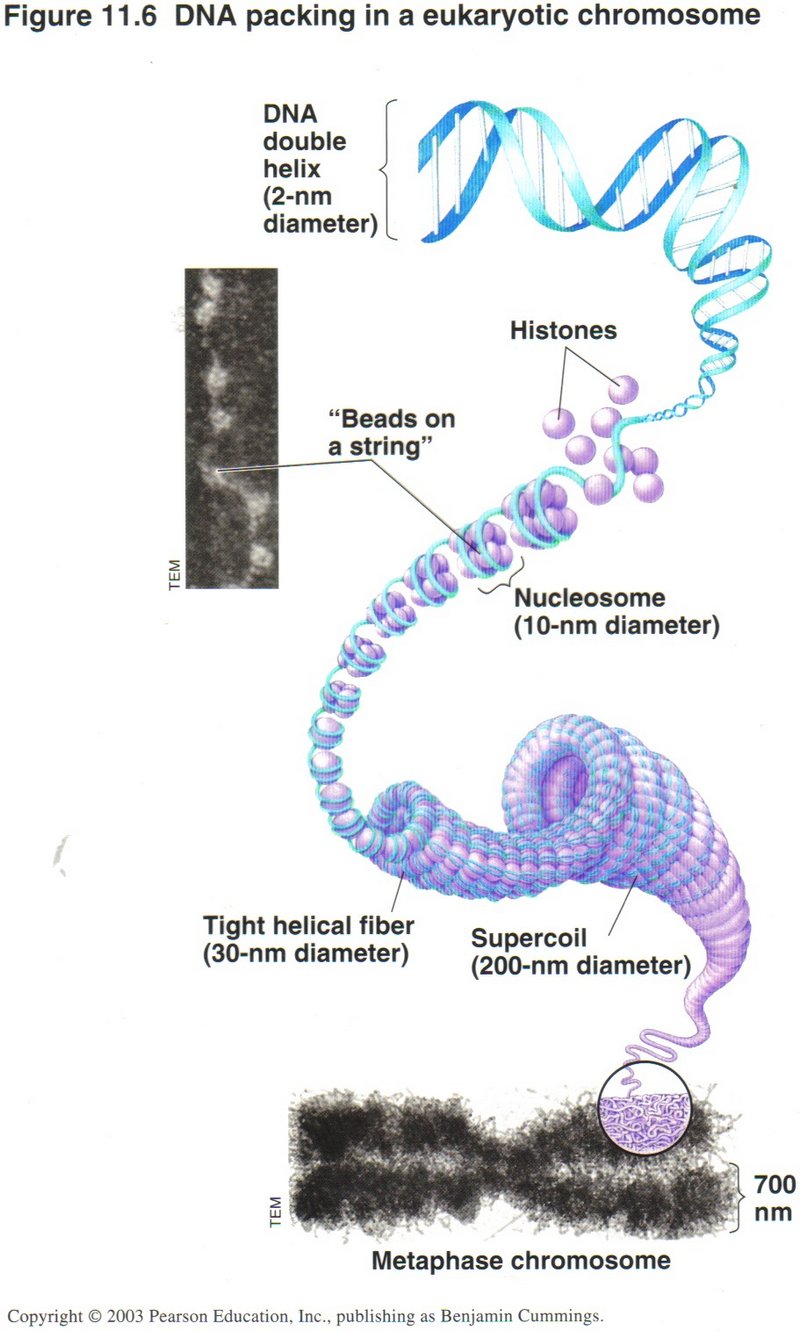
DNA in the nucleus is so long ― each molecule is from 500,000 to several million base-pairs (nucleotides) in length ― that
its handling by the cell machinery must be delicate indeed, as this view shows. Humans have 46 molecules (called Chromosomes)
totalling over one billion base-pairs. Since the molecule of DNA is so long, its handling requires an elaborate system of compression
to get into the microscopic dimensions of the cell. This is accomplished by multiple layers of folding. There can be up to four levels
of folding, the first being the helix. The secondary level is the winding of the helix about 8 proteins, called histones, as shown in
the center of the view. In some portions of the cell’s life, this secondary winding is coiled into a third level, and this in turn into
a supercoil (fourth level) when the cell is about to duplicate.
Click here for next page or click here for further description of the DNA helix.
DNA is involved in two major processes: replication (duplication) and transcription (the first step in
using the information it carries).
We will examine replication first.
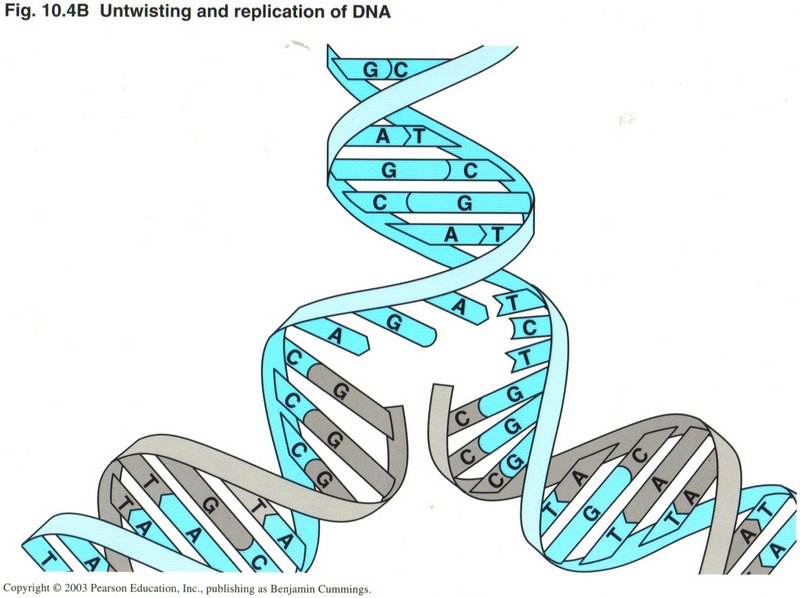
Replication (Duplication) of DNA
In this view, you can see how replication is accomplished. The parent DNA strands are separated, and simultaneously each strand is
fitted with its complement base ― "A" (adenine) with "T" (thymine) and "G" (guanine) with "C" (cytosine).
The two strands are now four, and properly pair off as duplicate daughter strands as they wind back into the two helices.
Click here for next page
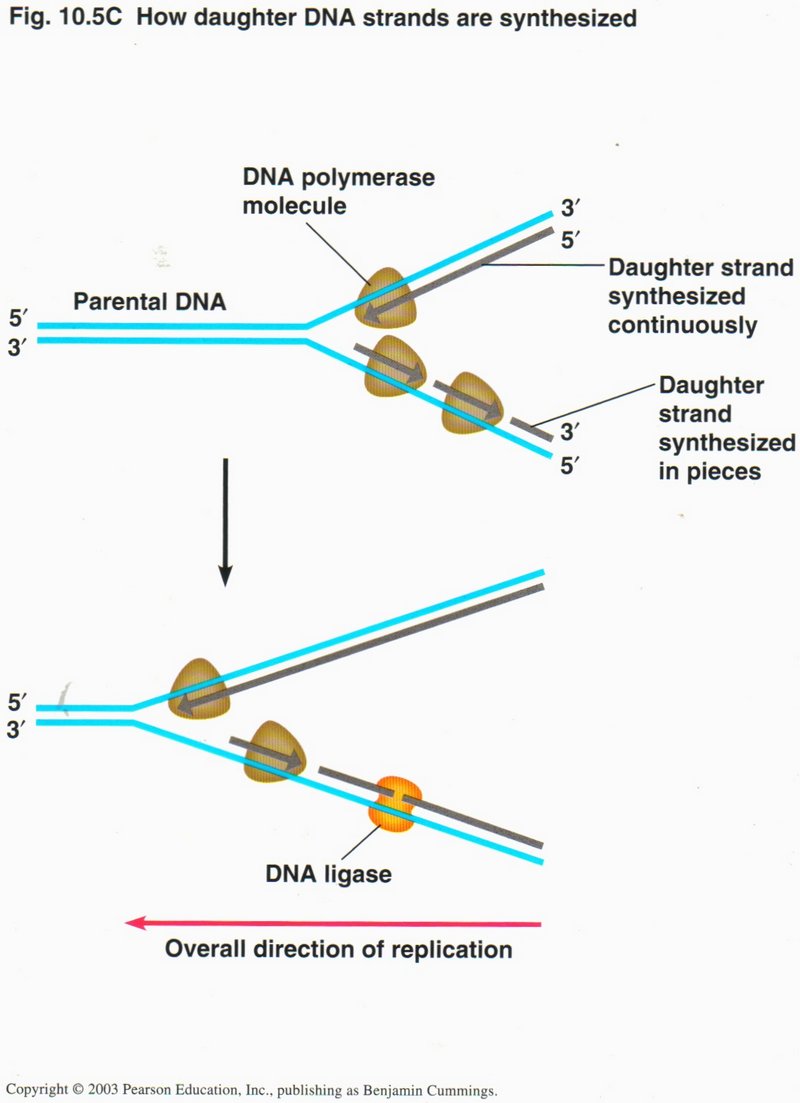
As this view shows, the actual operation is carried out by several protein complexes. These complexes are actually molecular machines,
whose function depends on their exact configuration. One (not shown) unwinds the helix. The second (DNA polymerase) carries out the
synthesis, creating a continuous daughter strand to the parent strand running in the 5’ to 3’ direction; but, since DNA polymerase can
synthesize only in the 5’ to 3’ direction, the synthesis of the opposing strand (that runs in the opposite direction) has to be
accomplished by short bursts by the DNA polymerase complex, but still in the 5’ to 3’ direction.
These separate short strands are sealed into one continuous strand by a third protein complex, called a DNA ligase. Although
not shown in these simplified drawings, the actual process requires an RNA starter section, because single-strand DNA is quite
unstable, particularly in short lengths. These RNA starter units are replaced by DNA in a separate operation called proofreading,
where still another protein complex checks the strength of bonding of each base to its partner, and removes and replaces any that are
not tightly bound.
Click here for next page.
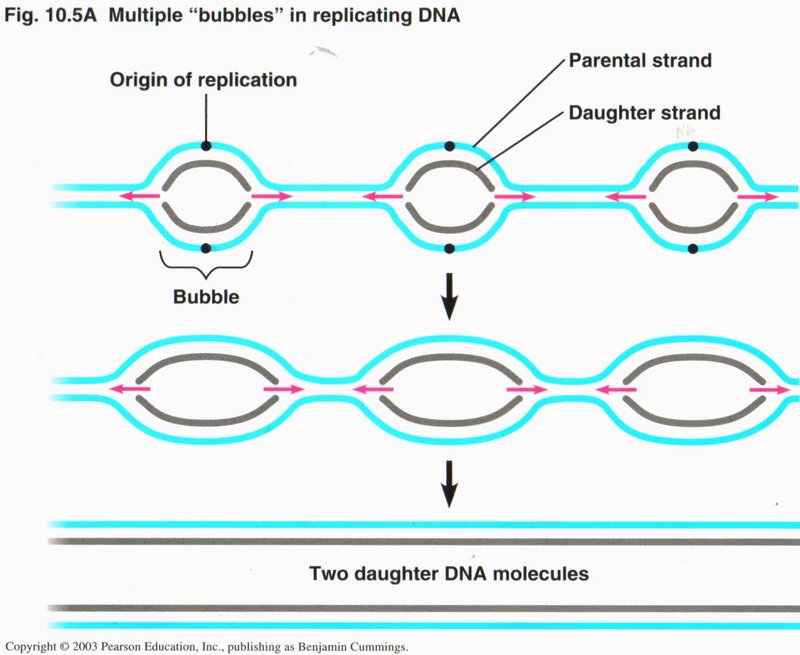
Replication is carried out in eukaryotic (cell having a nucleus) cells in multiple in each chromosome. Specific sequences of
nucleotides are recognized by the initiation proteins to determine where the origins are to be. In prokaryotic (cell without
a nucleus, i.e. bacteria) cells, there is only one circular molecule which is attached at one point to the plasma membrane, where
replication is initiated by two replication forks proceeding in opposite directions around the ring of DNA until meeting at the far
side.
Click here for next page.
Transcription and Translation
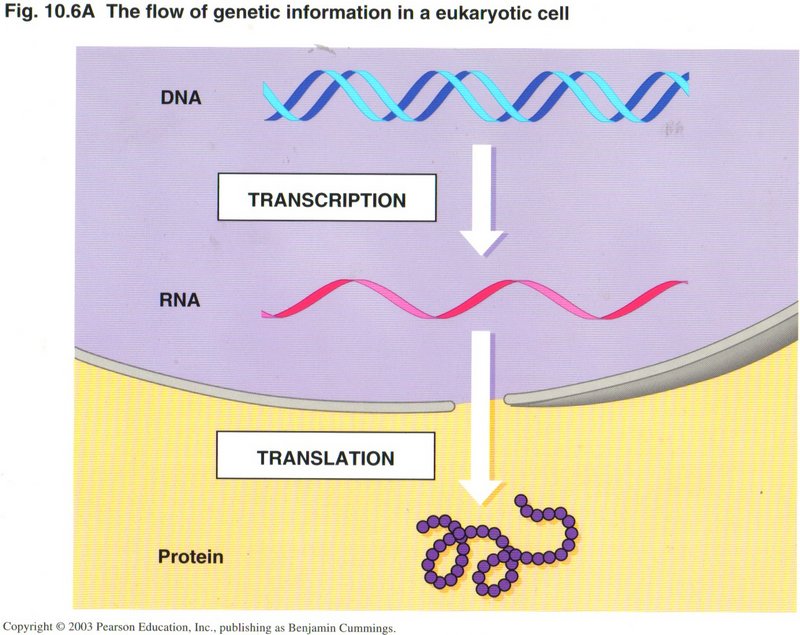
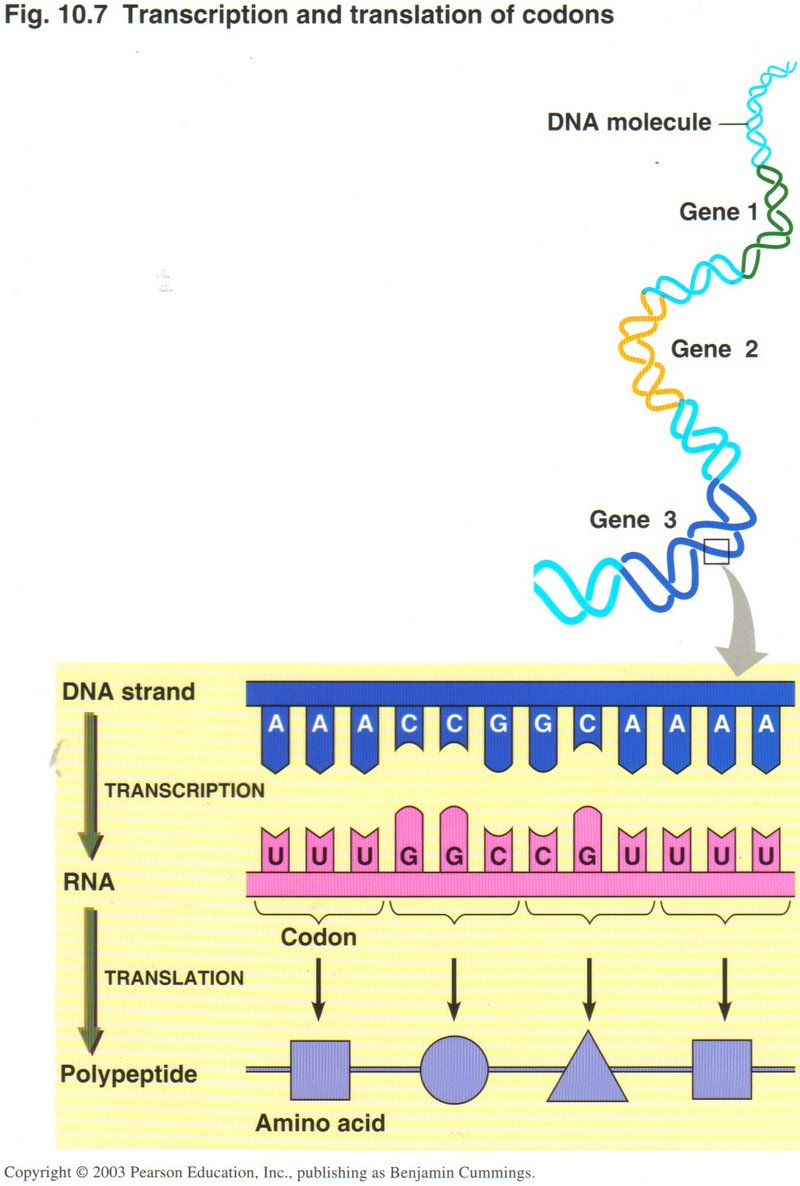
The processes of transcription and translation are schematically shown in these views. On the left is a simplified view, and on the
right a more detailed view, of these processes. Transcription results in the creation of a single strand of RNA (very similar to DNA),
of which there are several types. The type which is used in the process of translation to create a protein is called mRNA
(messenger RNA). The differences between any RNA and DNA are very slight: The sugar ribose (R) in RNA takes the place of the very
similar sugar deoxyribose (D) in DNA, and the base uracil (U) is substituted for the very similar base thymine (T). Why are there
two types of information molecule? RNA is stable in a single ribbon, convenient for translation into a protein, but not for the
lengths required to be the information storage for the whole organism. It is not stable in paired form (as is DNA), and thus could not
be duplicated as accurately as can DNA in replication. DNA is not stable in single-strand form, but is very stable in its helical form.
It is amazing how these small changes in composition could make such a great difference in the way DNA and RNA react to their
environments.
Click here for next page
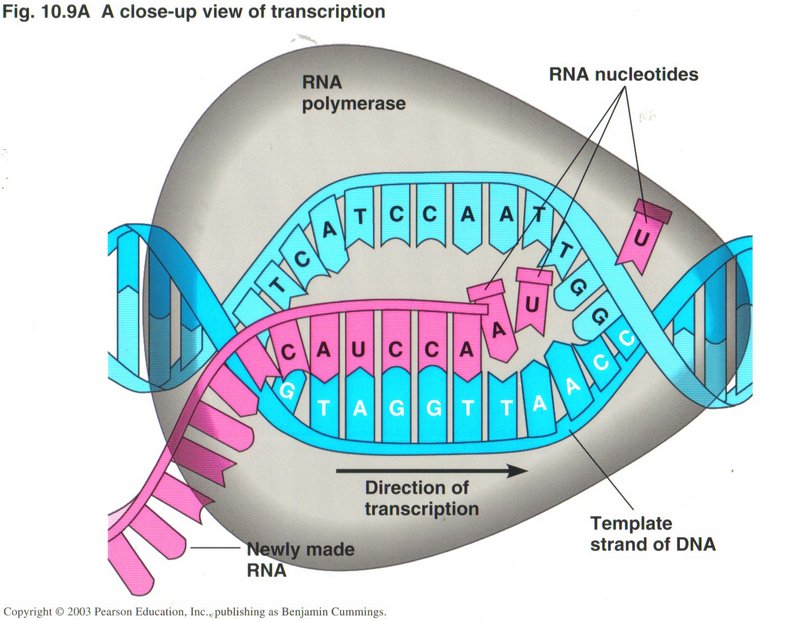
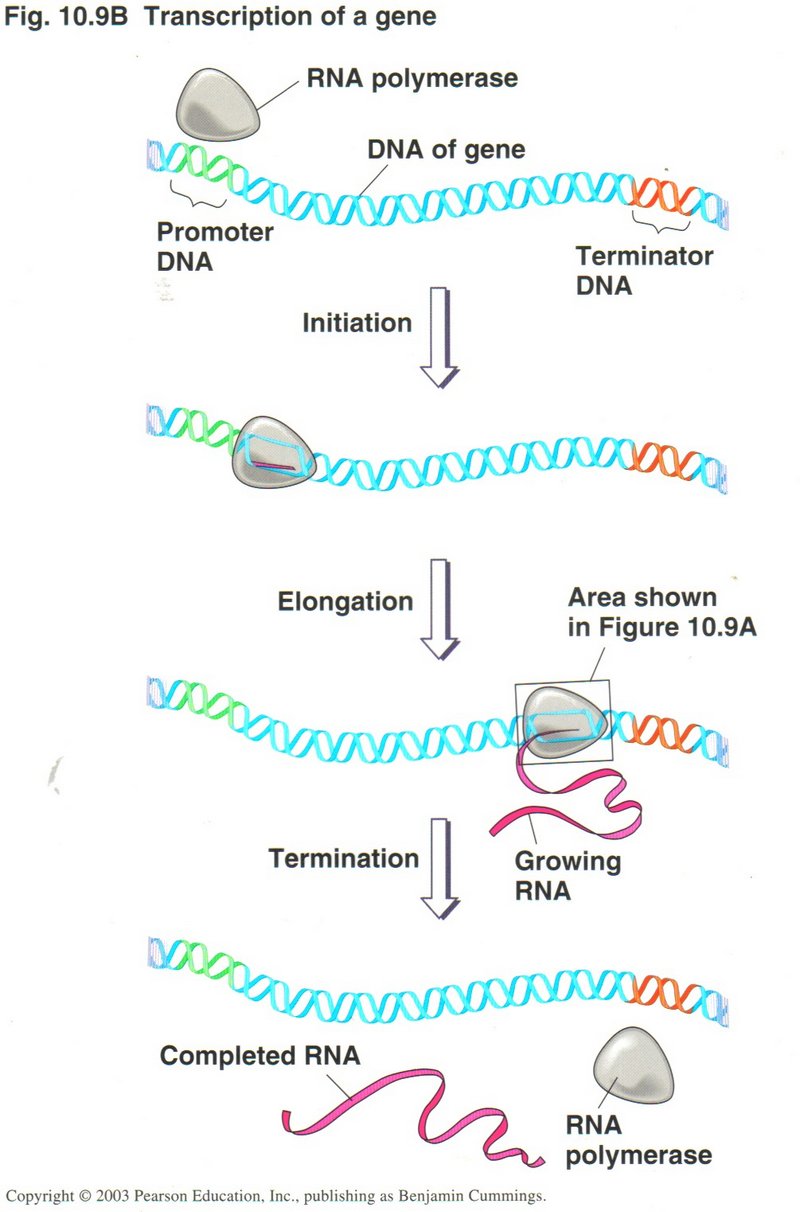
Here we see two
views of the process of transcription. The one on the left shows the whole process while the one of the right shows a close-up giving
more details of the process. One sequence of DNA base-pairs is selected on one strand of the DNA to produce
the mRNA with its sets of three base-pairs (called codons). A protein is simply a long string of various amino acids, twenty of
which are produced by translation of the mRNA. Since two base-pairs can only produce 16 different combinations, it requires three
base-pairs of four kinds to produce the 20 types of amino acids. But three base-pairs will give 64 combinations of the base-pairs,
forming 64 different codons, thus providing for "start" and "stop" codons and several ways to form some of the acids. Note that the RNA
string of bases is identical with the opposite DNA strand from the template strand (except for the substitution of uracil for thymine).
Click here for next page
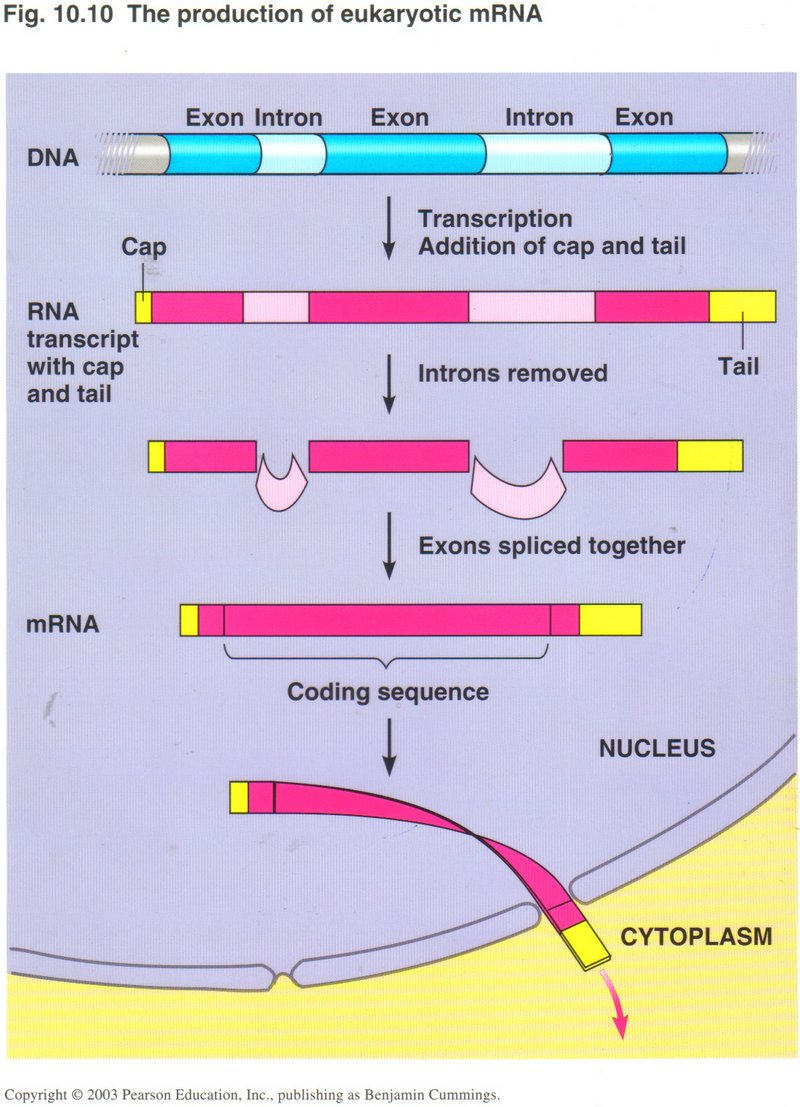
The mRNA ribbon requires several more steps before it is ready for the translation process. Here we see that the string of DNA base-pairs
that compose a gene may be split up into exons and introns. The exons are the true genetic base-pairs, whereas the introns
are “intruders”, sections of DNA that do not contribute to the gene expression, and must be removed. Also the mRNA molecule is fitted
with a cap and a tail to ensure that it will not be broken down by enzymes loose in the cytoplasm.
Click here for next page.
Translation of mRNA
Here we see the end product of transcription/translation ― the amino acid, a string of many of which constitute a protein. Although
more than 60 different types of amino acids are found in cells, only 20 are produced in the transcription/translation processes. These
20 are further limited to the so-called left-hand image version (shown in the picture) of those amino acids which can have a side chain
("R" in the view). A right-hand image version would have the amino group and carboxyl group reversed with respect to the "R" chain.
Click here for next page.
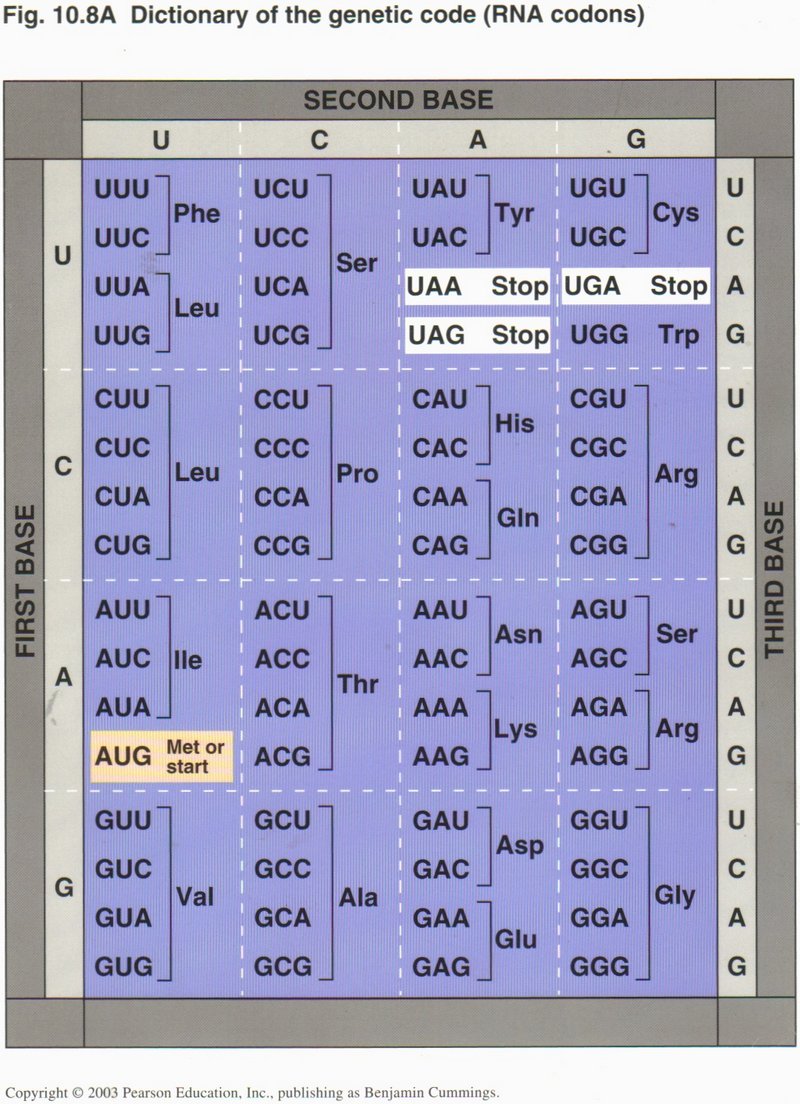
The 20 amino acids are related to the possible codons in the mRNA as shown in this chart. The amazing fact is that (almost) ALL living
things use this same arbitrary code to relate the DNA’s base-pair sequence to the protein’s amino-acid sequence (there are several
obscure exceptions). Note the specific codon for “start” and the three specific codons for “stop”, and the possible redundancies from
two to six codons for many of the amino acid designations. In fact, only two acids (Trp and Met) must be designated by a single specific
codon.
Click here for next page
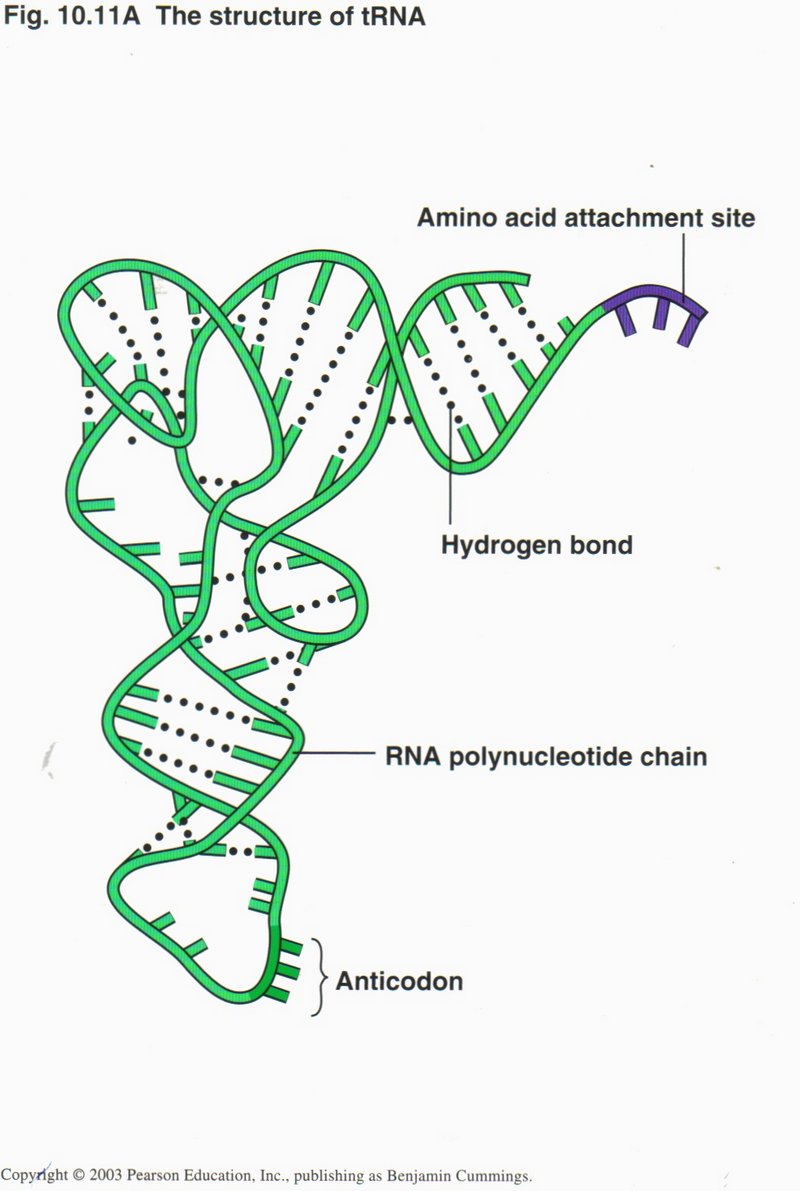
Another type of RNA, called transfer RNA or tRNA, is an essential intermediary between the mRNA and the protein. This molecule
is so structured that at one end it matches the conformation of a particular one of the 20 amino acids and at the other end matches
the codon representing that acid on the mRNA. Obviously, there must be and are 20 types of tRNA.
Click here for next page
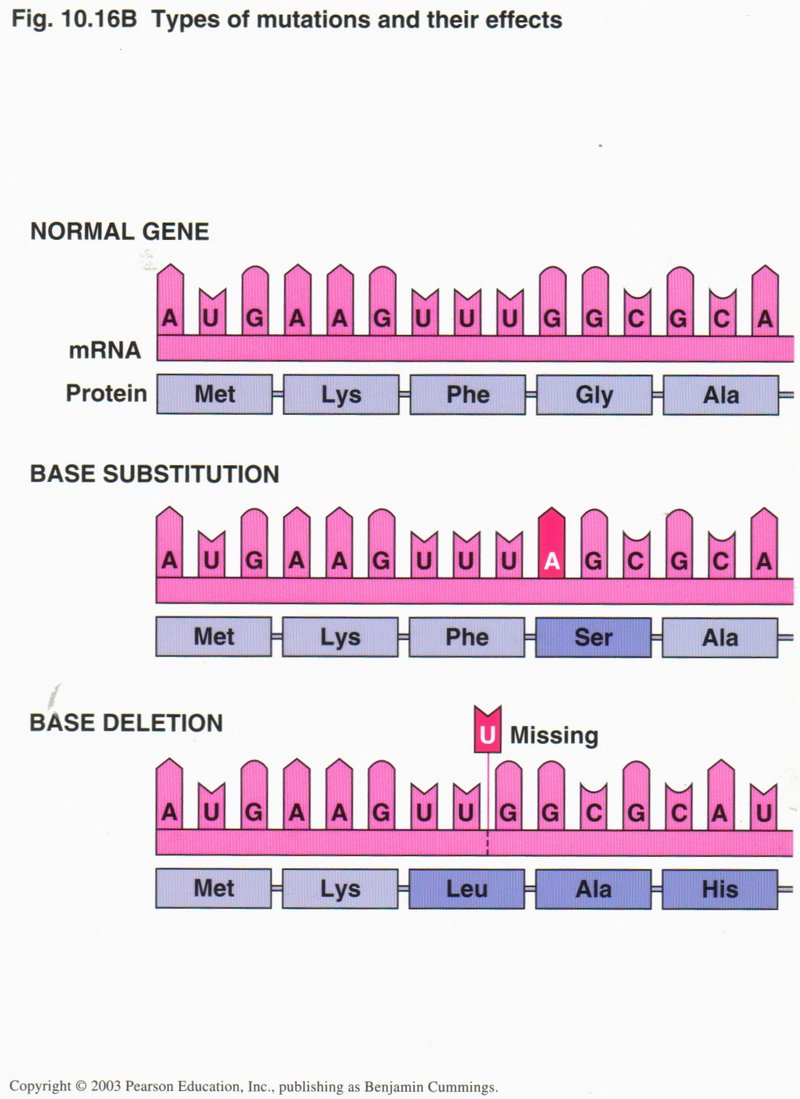
Translation can involve errors, called mutations, as shown in the view on the left. In the base substitution mutation, one
nucleotide in the RNA has been incorrectly transcribed or else the DNA template is in error from a previous replication. The wrong amino
acid serin (Ser) will be selected by the mutated codon, rather than glycine (Gly) which was originally coded.
In the base deletion mutation, a nucleotide was omitted in the translation from the DNA gene, and all amino acids following
will be different from the ones intended by the gene DNA.
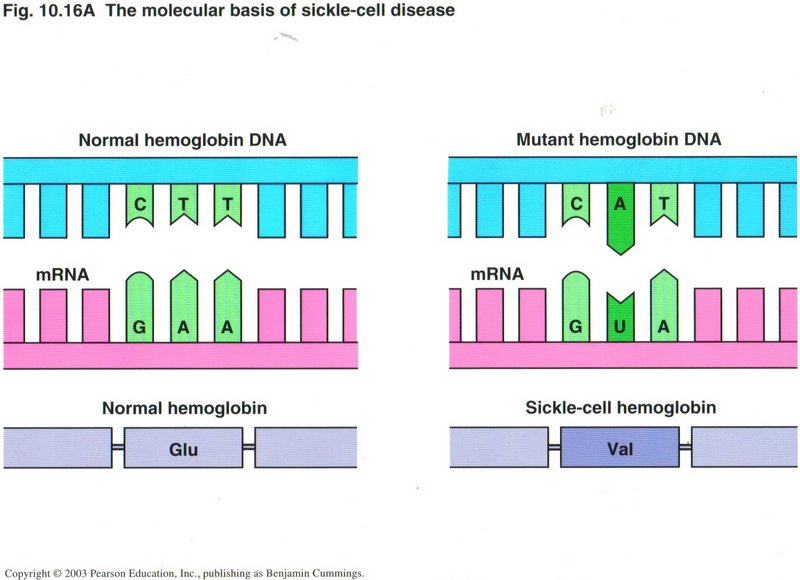
Usually the first error is not critical ― but can be, as in the case of sickle cell anemia (see right) ― but the
second is usually disastrous, as the resulting protein is usually nonfunctional.
Click here for next page
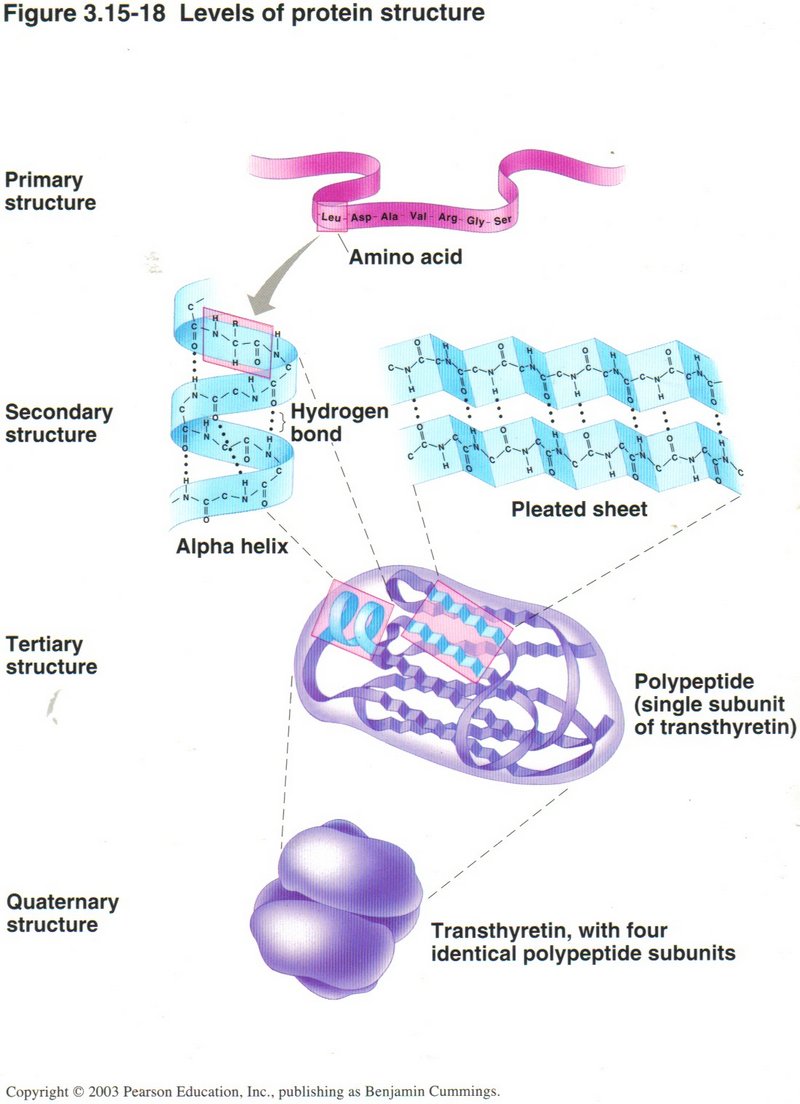
Production of a polypeptide (protein) is not complete with the translation process. Many polypeptides must undergo chemical modication
and folding before they can be called proteins. Most proteins must be folded into at least a secondary structure, and many must be
further folded into a tertiary structure, which must have the exact shape required for its binding to DNA or to another protein, and
some even require folding into a quarternary structure to form part of a protein complex, as shown at the bottom of this view.
Click here for next page or Click here for further details of translation.
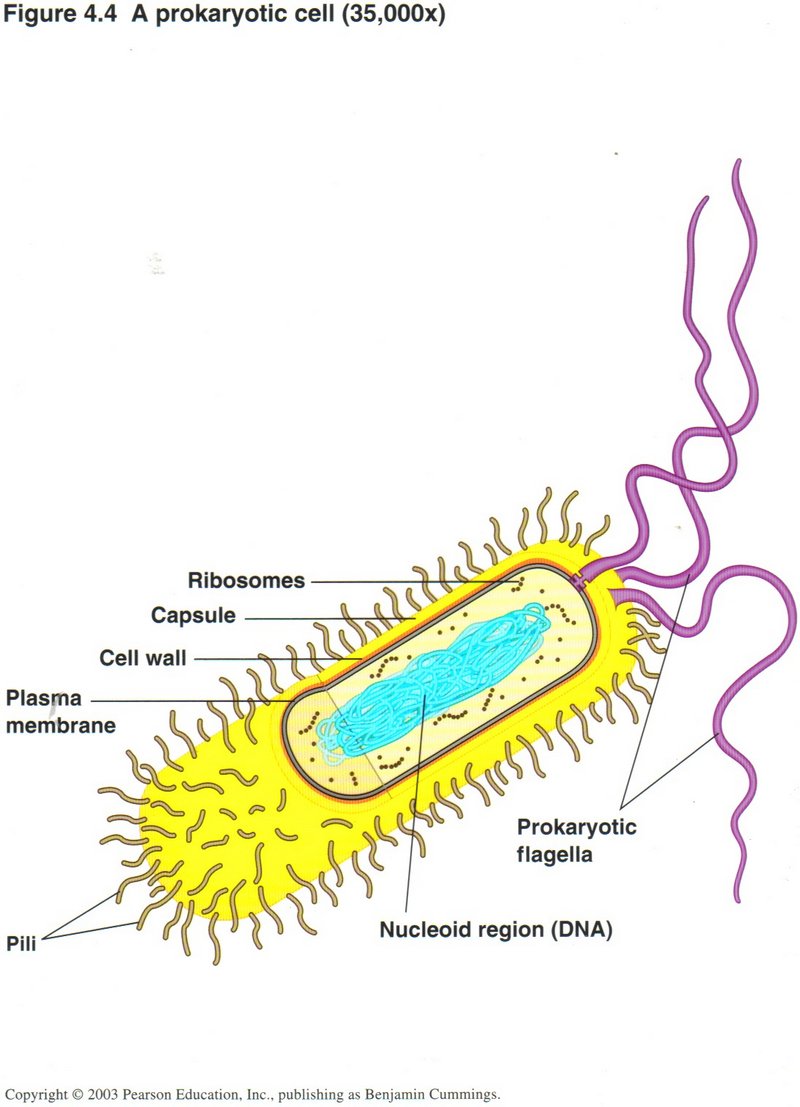
Bacterial (Prokaryotic) Cells
Although the illustrations shown above largely involve eukaryotic cells, all the functions described also take place in the bacterium
(prokaryotic) cell. As shown, the cell is encased in a capsule, and protected from the outside environment by a cell wall/plasma
membrane casing, containing suitably controlled pores to admit and release certain substances necessary to the cell’s life. Within
the plasma membrane is the cytoplasm, the fluid that contains all of the cell’s components ― its DNA, its ribosomes, its
chemical factories for producing amino acids, nucleotides, ATP for energy, and whatever other substances the cell needs, as well as
other areas where complex molecules are broken down into simpler ones for use in the chemical factories. The absence of introns in
the DNA of bacteria is one difference between the DNA of bacteria (prokaryotes) and the DNA of all the other kinds of life (eukaryotes).
Another difference is that the bacterial DNA is almost always in a circular form (one molecule), while that of the eukaryotic cell
is linear (several to many molecules called chromosomes). The working proteins are slightly different in the two types of cell,
but the basic processes are almost identical.
Click here for next page
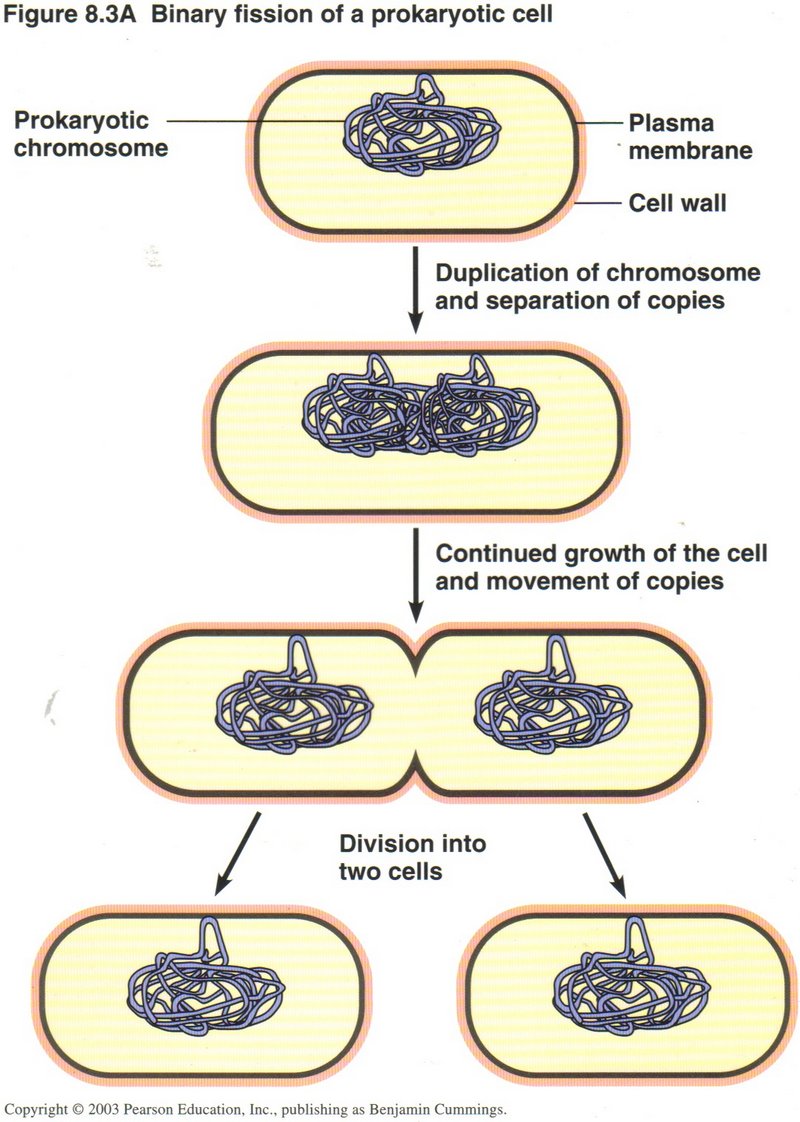
Before dividing, a bacterium must manufacture a duplicate for every molecule in its cytoplasm ― DNA nucletides, ribosomes, chemical
factories, and breakdown factories.
Click here for next page
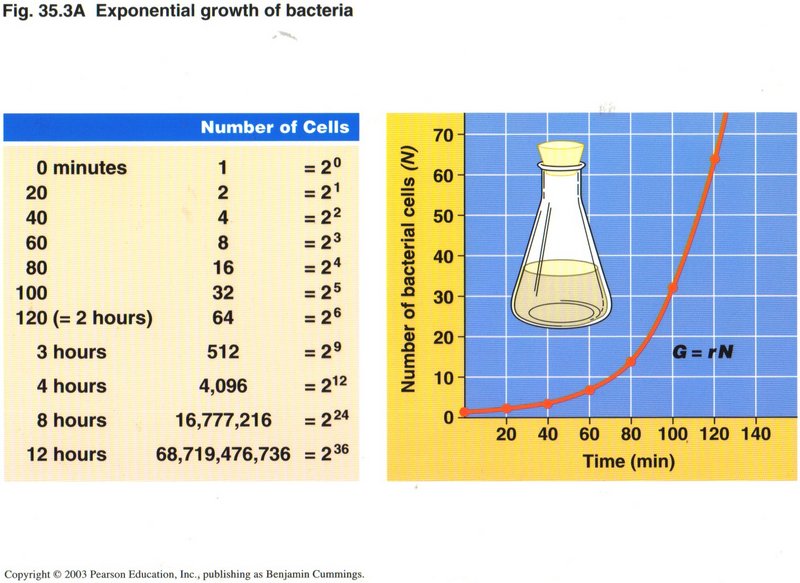
Fission can be frequent ― as often as every 20 minutes if sufficient nutrients are available.
Those scientists who insist that life arose from purely natural causes ― without supernatural intervention ― are hard pressed
to explain the origin of multicellular life. After all, bacteria were doing quite well. For at least 2,000 million years they were
probably the only form of life. No doubt there arose a number of specialized forms of bacteria, as we see today ― but there was no
evolutionary need for anything else. In fact, the type of cell needed for the specialized anatomy of advanced plants and all animals
was far more complex than the prokaryotic cell of the bacteria.
Click here for next page
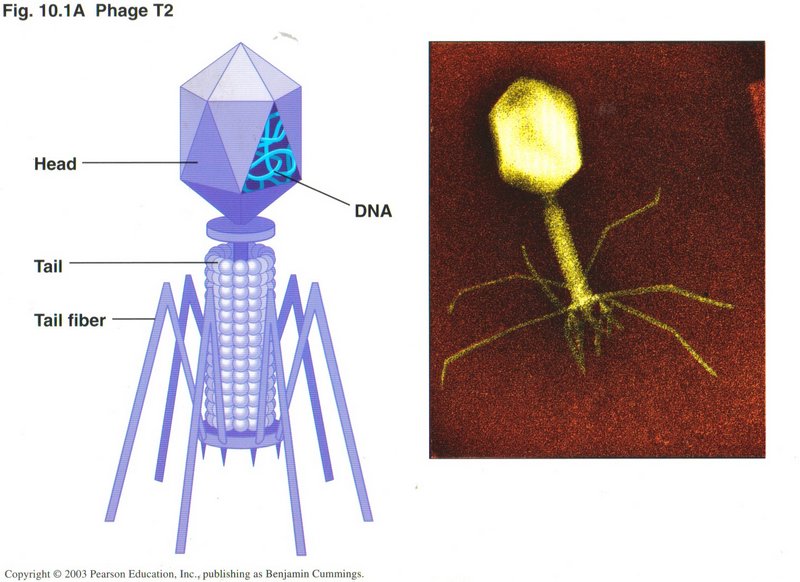
Viruses
As you probably know, viruses don't qualify as living things, because they cannot reproduce themselves. Instead, they invade cells of
some form of life and use that cell's machinery to reproduce the virus. A typical virus is the T2 phage, which attacks bacteria. Here we
see an artist’s conception and a microphotograph of a Phage T2 virus. Note that the principal content of the head of the virus is DNA.
We will look at the HIV virus which causes AIDS in the Third Session.
Click here for next page
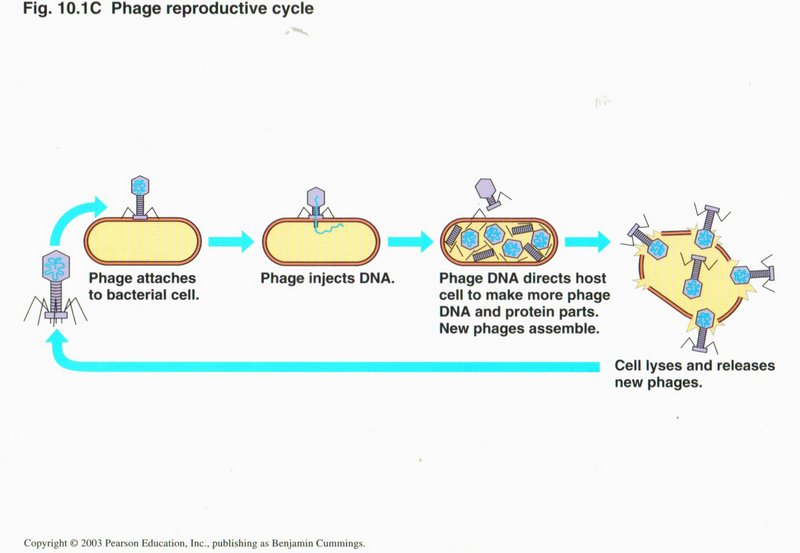
In this view are the four stages of a virus attack on a cell. (1) After finding a cell to which it can bind at one of the cell’s
recognition sites, the virus attaches itself. (2) The virus injects its own DNA and possibly some specialized proteins into the cell.
(3) Using the cell’s own machinery, the viral DNA reproduces its components many times, including the proteins to assemble the virus.
New phages assemble. (4) The production of so many viruses causes the cell to burst, killing itself and spreading hundreds more
viruses into the extracellular medium.
Click here for next page.
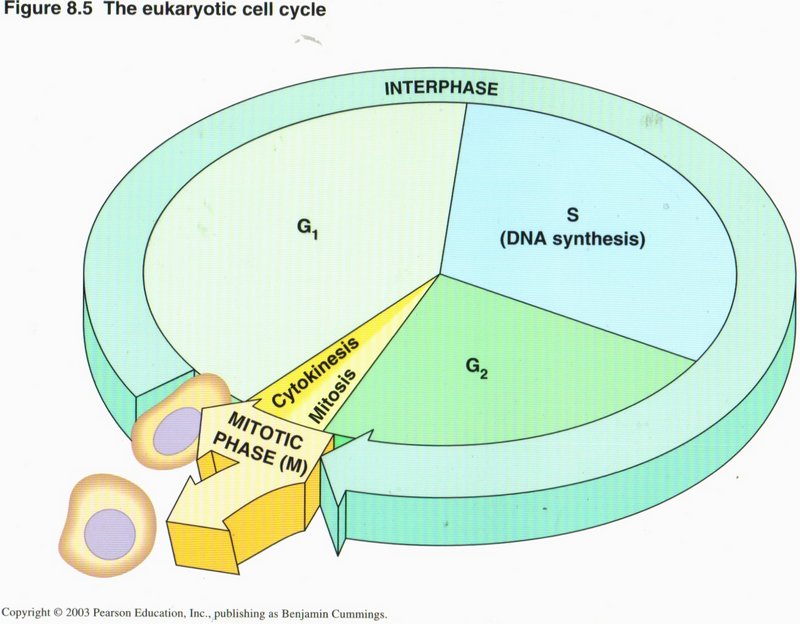
The Cell Cycle
The cell’s life cycle is charted in this view. The period when only normal activities occur (no duplication) is called G1, and
gene expression leading to RNA and protein formation goes on continuously. When triggered to do so (described below), the cell enters its
S phase, during which DNA replication (duplication) takes place, along with normal activities. When duplication is complete, the
cell enters the short G2 phase, when normal activities shut down, and the cell’s machinery concentrates on producing all the
duplicated organelles and their contents as well as the special proteins required to carry out the forthcoming cell division. This latter
process is carried out in the relatively brief M phase wherein either mitosis or meiosis (governing the handling of
the duplicated DNA helixes, described below) and cytokinesis (governing the separating of the other duplicated organelles and
actual separation of daughter cells) take place. The daughter cells immediately enter their G1 phase.
Click here for next page.
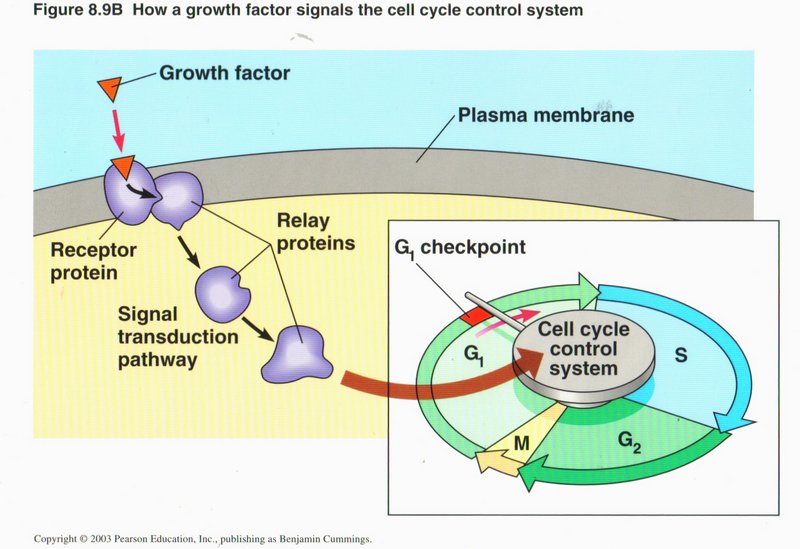
The four phases each have check points at which signals must be given before the phase will proceed. As shown here, the G1 checkpoint is
triggered by relay proteins activated by a growth factor from another part of the organism. Other checkpoints may originate internally,
to insure adequate materials are on hand for the next events. It is when these signals become deranged by DNA mutations, that cancer
(uncontrolled cell division) develops.
Click here for next page or Click here for a more detailed study of mitosis and meiosis.
Mitosis and Meiosis (Creation of Sex Cells)
Most of the cells of the organism are non-sex cells which separate in the process of mitosis and generate two identical daughter cells.
But advanced plants and animals divide their sex cells in the much more complex process of meiosis, wherein a cell containing two sets
of DNA (called diploid) divides into four grand-daughter cells, each containing one set of DNA (haploid cells). The
process of meiosis is different for male parental cells than for female parental cells, but each yields four grand-daughter cells.
These processes take place in cell cycle phase S.
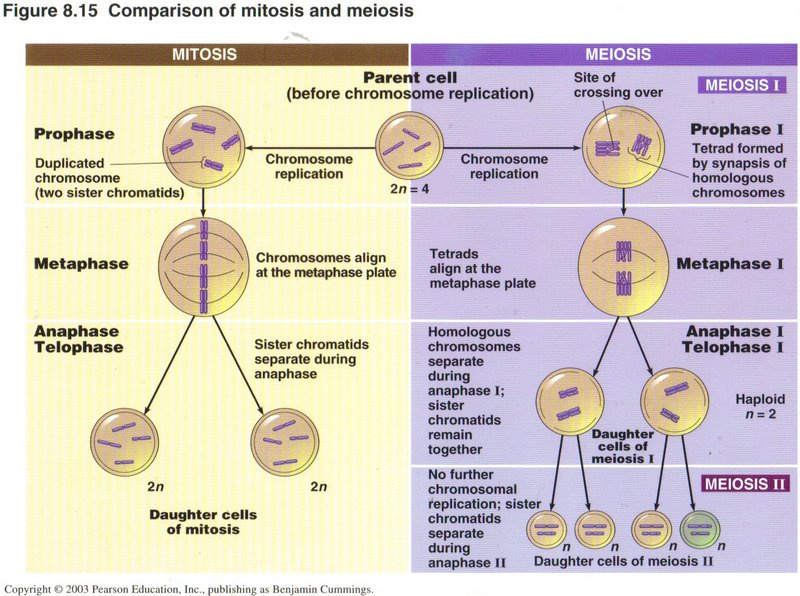
In mitosis (see left column) the two similar chromosomes ― one from the father and the other from the mother ― are
treated as two independent chromosomes, and each is duplicated into sister chromotids. In prophase, these pairs of chromotids
are independently condensed into rod-like structures and tied together as sister chromotids. In metaphase, the chromotid pairs
are lined up in the center of the nucleus, separated, and drawn to opposite ends of the cell. In anaphase and telophase
the cell divides into two cells with complete complements of all of the original cell’s contents, including the DNA.
Click here for next page

In meiosis (see right column), however, during prophase I, the two chromotids from the male parent’s chromosome are joined with
the two chromotids of the corresponding chromotids from the female parent's chromosome to give four similar chromotids. During this
process one or more chromotids may “cross over” another chromotid, and in the process of straightening out the tie-up one or more genes
from one parent may be placed in the corresponding gene sites of the other parent, creating a mixing of similar genes from the two
parents. Occasionally the repair process splits parts of genes and thus usually invalidates that gene in the organism inheriting it.
This splitting is the cause of many defects in new-born babies.
In metaphase I, the four chromotids are collected at the midpoint of the cell, as in mitosis, but any two of the four may go to one
pole with the other two going to the other pole. This obviously causes a major scramble of the genes from the two parents, so that
the resulting sex cells (called gametes) from thousands of such divisions may be all different. This is the primary mechanism
for spreading the gene pool of the organism into the population, so that all possible variations in the gene pool are expressed in
one or another of the population. This obviously confers survival benefits when environmental changes occur, as the likihood of some
of the population to survive is far greater than if the entire population were homogeneous.
Anaphase I and telophase I are quite similar to the corresponding phases in mitosis, but differ in the female host in that most of
the cytoplasm goes into one daughter cell and only a very small amount goes into the other.
Similarly in the second round of division phases, the male cells from the first division give rise to four similar cells in the
second division, but each such cell contains only one chromotid (now one or more chromosomes) and is therefore haploid. The
daughter cell from the female host which has the most cytoplasm produces two granddaughter cells, again with one containing the most
cytoplasm, which will become the female “egg.” The other grand-daughter cell along with the two grand-daughter cells from the
cytoplasm-poor daughter cell are not viable and are soon destroyed.
It is from mistakes made in meiosis that heritable diseases and child defects arise. Mistakes made in mitosis affect only the
individual involved, since the mistake is not passed on to other generations.
Click here for next page
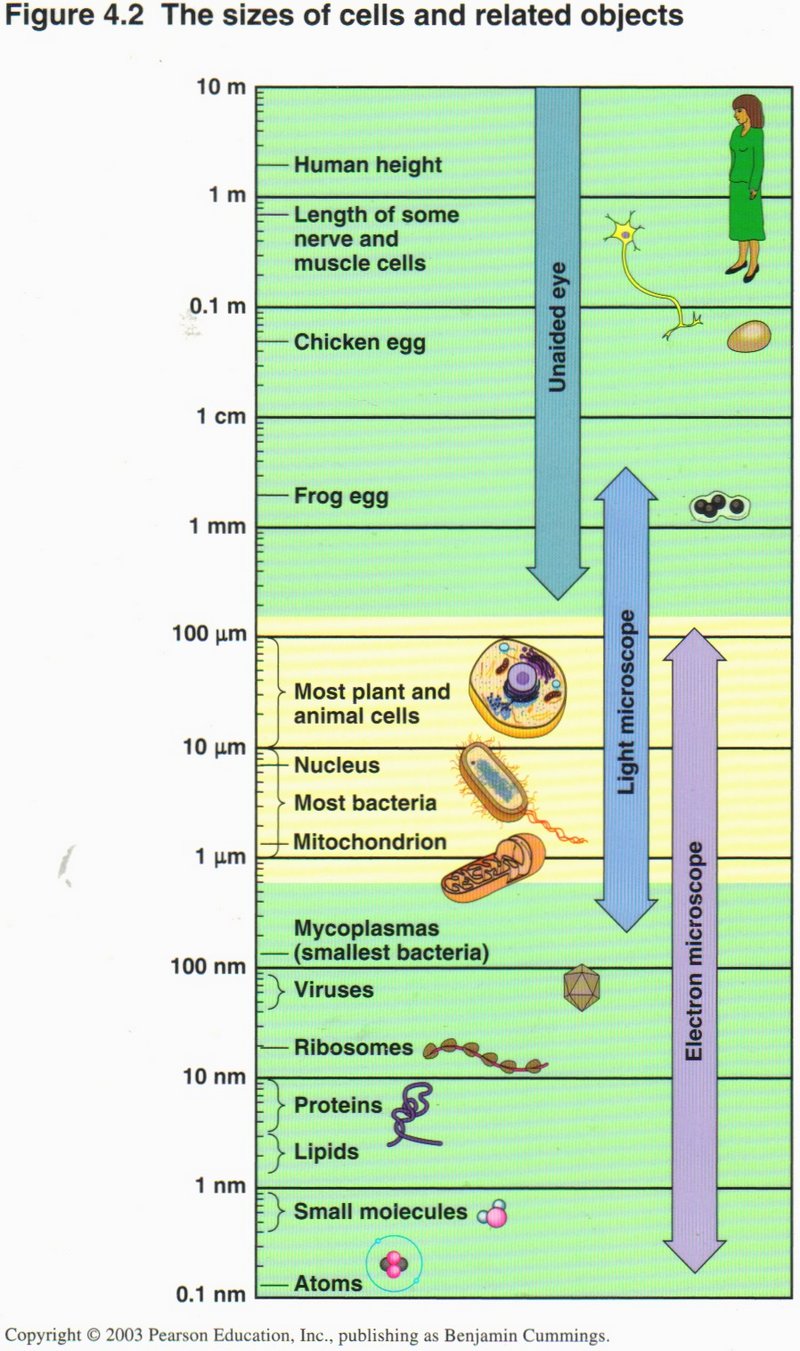
This chart may help to put the sizes of the cells and related objects into perspective. Each line from top to bottom representates a
reduction of 10 to 1 in size, so that the bottom line representes a size only 1/100,000,000,000th (that is one hundred-thousand-
millionth) as large as the top line. You see that while giant cells such as the nerve axon of the giant squid and eggs of familiar
animals are visible to the naked eye, most cells can be seen only in the microscope and even the electron micoscope is taxed to show
the virus, a ribosome, or some cell organelles. Cells vary in size over a range from a million to one.
This highly over-simplified account of the highly complex processes in advanced cells is given to emphasize anew the amazingly
beautiful design our Creator used in producing living things. Most of the processes are interrelated, and the molecular machines that
carry them out must be completely and correctly assembled from their (often many) constituent parts before they can perform their tasks.
This design is still more evident in the examination of higher organisms, including man, which will be undertaken in the Third Session.
We have presented this complex subject to you, not to educate those of you who are not versed in the biological sciences, but
simply to show how unfair it is to our Creator to think that these tiny cells, so small that only the largest can be seen with the
naked eye, and the smallest stretches the power of even the electron microscope, could have arisen by blind natural processes.
Resources Usd in Preparation of This Session
(1) Biology: Concepts and Connections (Third Edition), by Neil A.
Campbell, Lawrence G. Mitchell, and Jane B. Reece, a university biology
textbook. Published 2000, by Benjamin Cummings.
(2) Cell and Molecular Biology, by Stephen L. Wolfe, a university biology
textbook. Published 1995 by Wadsworth Publishing Company.
(3) The Genesis Question, by Hugh Ross. Published 1998 by NavPress.
(4) Genomes, by T. A. Brown, a university textbook. Published 1999 by
John Wiley & Sons.
Go to Third Session.
Return to Herb's Place
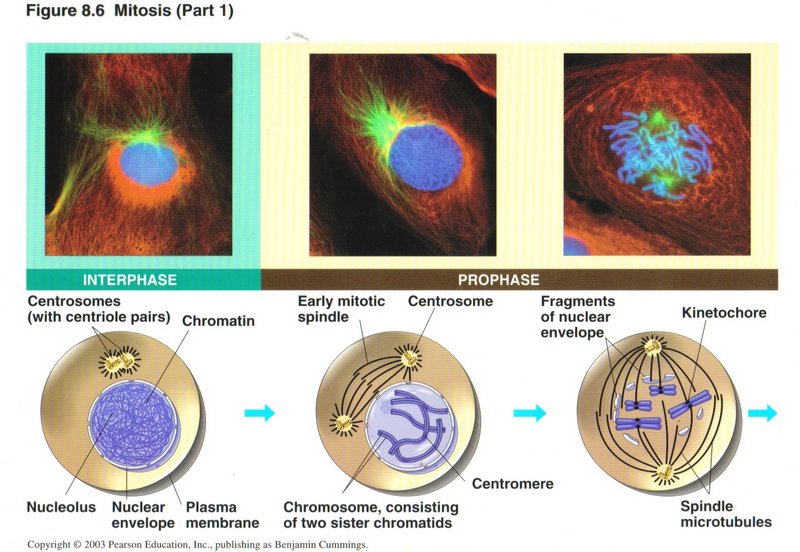
Mitosis
Mitosis is the mode of division of somatic cells (all cells except sex cells) of advanced plants and animals. All of the original
parental chromosomes are treated alike, so that the two daughter cells are identical. Mitosis is divided into four phases, the first
(called prophase) being shown here. At the beginning of prophase the DNA is loosely organized in its second level of folding
(being wrapped around histones, but not otherwise folded), as this is the form in which transcription is expedited. Each DNA molecule
is called a chromotid, and the entire genome DNA is referred to as chromatin (left view). There are two centrosomes which migrate to
opposite ends of the cell during prophase and extend microtubules to the center of the cell, forming the network of microtubules
called the spindle. Each chromosome has now been duplicated and consists of two sister chromotids, each folded into a third level coil
and that into a fourth level supercoil ― such a compact form that the chromatids are visible in the light microscope as rod-like
entities. (This is the only time the DNA molecules are technically called chromosomes.) The two sister chromatids are held together at
a region of the DNA molecule called the centromere by a protein complex called the kinetochore (center view). When the spindle has
formed, the kineochores anchor to a microtubule (right view).
Click here for next page
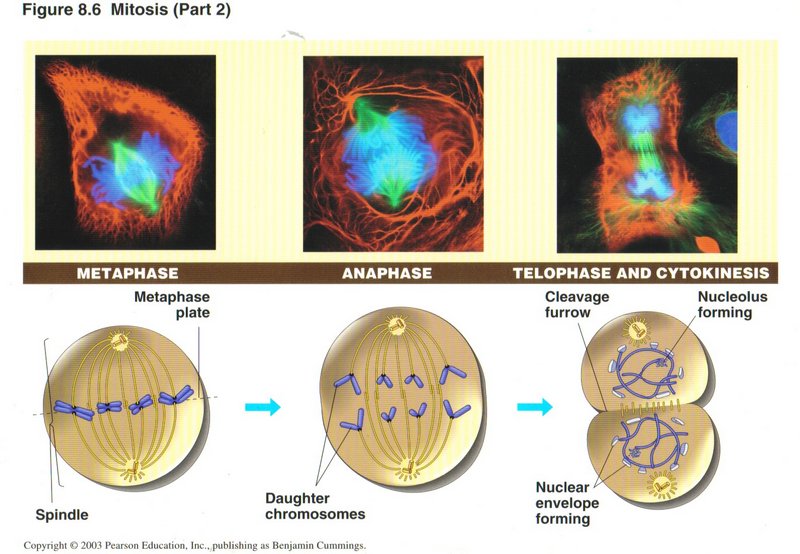
In metaphase (left view) the sister chromatid pairs migrate to the center of the spindle. The kinetochore connects one sister
chromatid to the microtubule going to one pole and the other sister chromatid to a microtubule going to the other pole. (The
microtubule structure is such that the two directions are identifiable by the kinetochore.) During anaphase the microtubules
disintegrate, drawing their attached chromatids to the appropriate poles. During telophase, the chromatids revert to their second
level folding mode and begin transcription and in the simultaneously occurring cytokinesis phase the rest of the cell assembles its
duplicated organelles into the two halves of the cell, which then experiences a cleavage around the center of the spindle, during
which the plasma membrane expands to cover each half of the original cell, thus creating two cells, which immediately enter interphase
(G1).
Click here for next page
Meiosis
In sexual plants and animals there are two sets of chromosomes, one inherited from each parent. The cells are accordingly called
diploid. During interphase, the chromatin behaves as in somatic cells, allowing transcription of all genes to be normally
transcribed. In meiosis the sex cells undergo a significantly different series of events from those of mitosis, involving two
successive cell divisions, thus producing four granddaughter cells ― all different. The first division, referred to as meiosis
I passes through the same named stages as in mitosis, but different things happen. The second division, called meiosis II is quite
similar to mitosis.
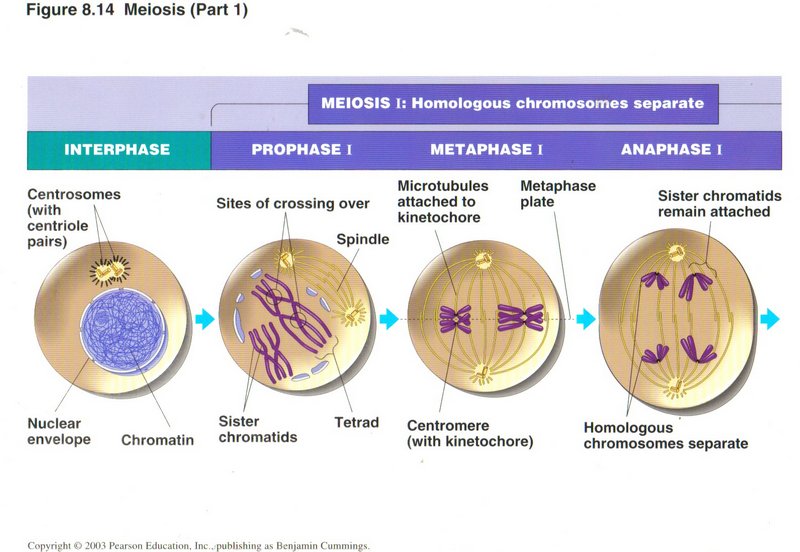
In prophase I, as the spindle is generated, the two duplicated sets (one from each parent, called a homologous pair) of each chromosome
are brought together, making four sister chromotids. In so doing, one or more chromotids may get entangled with another, a situation
called crossing over. Although a special protein complex attempts to straight out the mixup, occasionally errors are made, giving rise
to a number of physical deficiencies in the persons inheriting such cells. One type of error transfers a gene from one parental
chromosome to another, usually without any deleterious effects (shown and described below). But a second type of error causes only a
part of a gene to be so transferred, resulting in two defective genes. Depending on what the genes control, the error may be serious
or fatal, or it may be trivial.
In metaphase I, the two homologous pairs of chromotids are moved to the center of the spindle, where the kinetochore connects one pair
at random to a microtubule going to one of the poles and the other pair to a microtubule going to the other pole. Thus the chromosome
mix in each daughter cell is a random selection from one or the other parent. In anaphase I, the microtubule disintegrates, carrying
the attached chromatid pair to its pole. In telophase I and associated cytokinesis I the original cell divides into two daugter cells.
Click here for next page
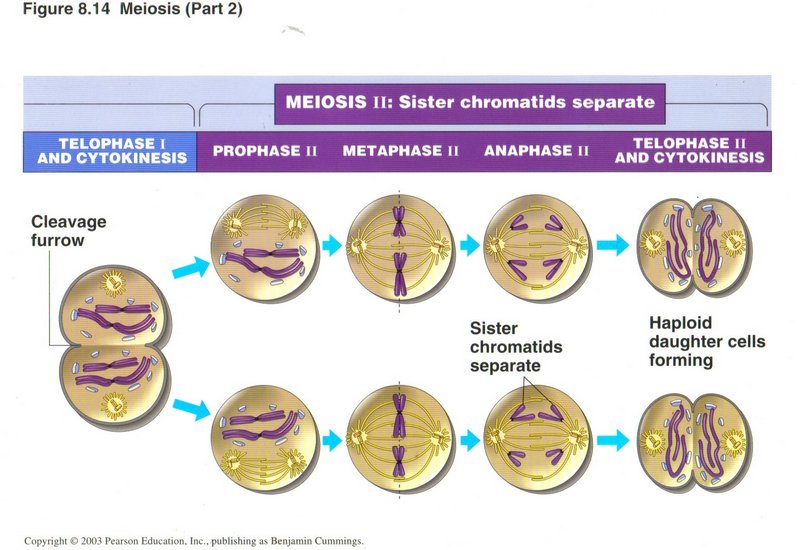 The diagram illustrates meiosis II for a cell from a male organism; if the parental cell was from a female, cytokinesis I would have
placed most of the cytoplasm and organelles into one daughter cell and very little into the other. The phases of meiosis II are quite
similar to mitosis, the exception again being different cytokinesis for the granddaughter cells of the original female sex cell. The
daughter cell that got most of the cytoplasm divides with most of that cytoplasm and organelles going to one grand-daughter cell and
little to the other. The deprived daughter cell also divides, but the three depleted grand-daughter cells are subsequently destroyed,
leaving only the one cell which becomes an egg cell. In both the male and female types of meiosis, the grand-daughter (haploid)
cells are called gametes.
The diagram illustrates meiosis II for a cell from a male organism; if the parental cell was from a female, cytokinesis I would have
placed most of the cytoplasm and organelles into one daughter cell and very little into the other. The phases of meiosis II are quite
similar to mitosis, the exception again being different cytokinesis for the granddaughter cells of the original female sex cell. The
daughter cell that got most of the cytoplasm divides with most of that cytoplasm and organelles going to one grand-daughter cell and
little to the other. The deprived daughter cell also divides, but the three depleted grand-daughter cells are subsequently destroyed,
leaving only the one cell which becomes an egg cell. In both the male and female types of meiosis, the grand-daughter (haploid)
cells are called gametes.
Click here for next page
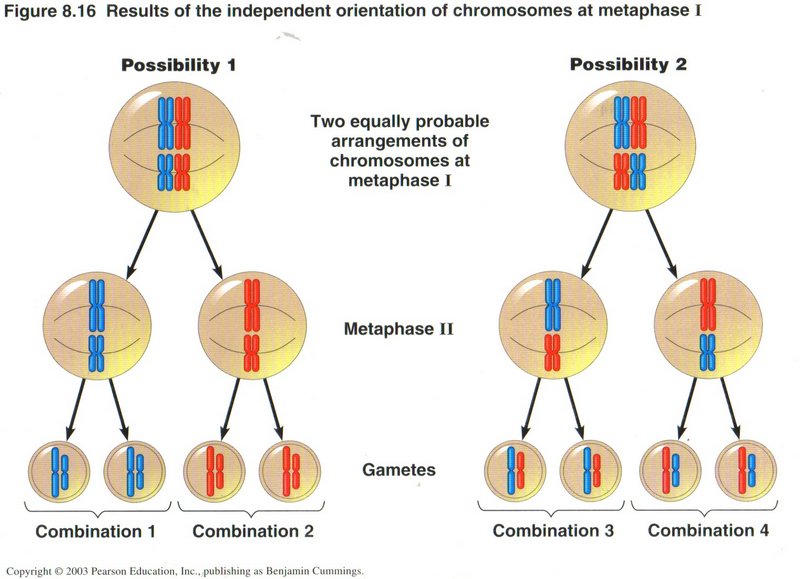
Here we see the equally probable results of chromosome mixing without cross over. We can expect the same number of each of the four
possible combinations of the original chromosomes to occur in any significant fraction of the population. This will be true for each
successive generation, and for all of the chromosomes of the particular organism.
Click here for next page
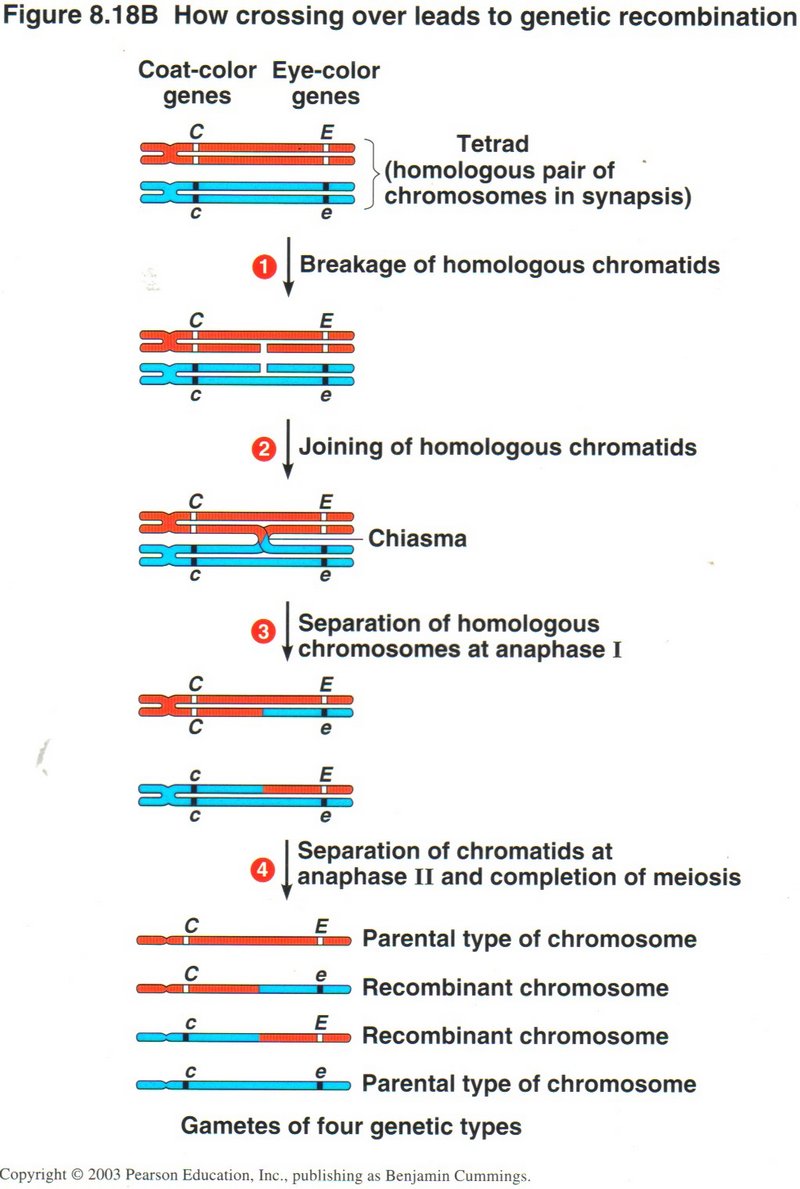
Crossing Over
In this view, we see a tetrad of chromosomes, red from one parent and blue from the other, with dominant coat-color gene C and
eye-color gene E from the red parent, and recessive genes c and e from the blue parent. (1) The crossing over repair required breaking
the inner red and blue chromosomes as shown. (2) The chromosome pieces are joined to opposite parental chromotids, an error called
chiasma. (3) The chromosomes are separated at anaphase I with the mismatched pieces including the red E and blue e genes. (4) The
resulting four gametes have all possible combinations of the original two pairs of genes.
Click here for next page
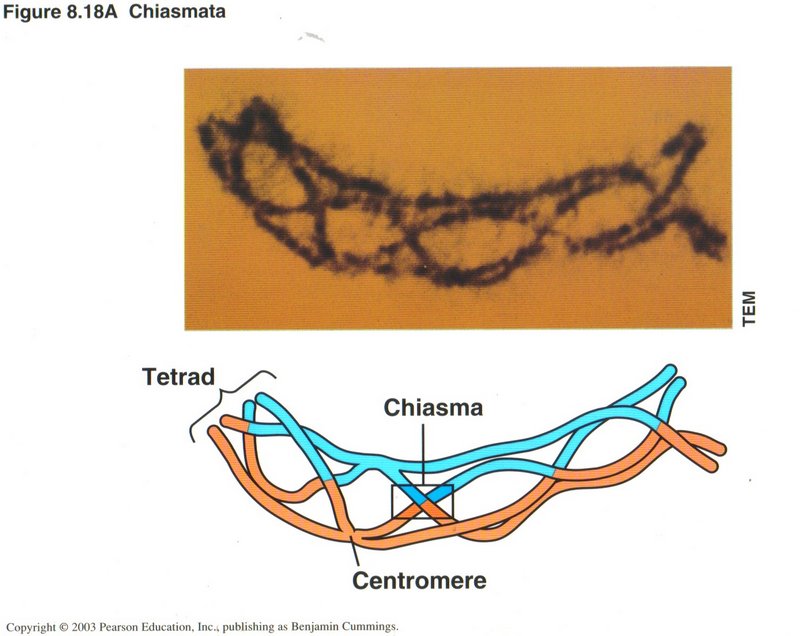
This view illustrates chiasma, the mismatched repair of a cross over. There are five chiasmatae in this example, any of which could be
partial gene cross over, with the probability of birth defect(s) in the person inheriting the partial gene(s).
Click here for next page
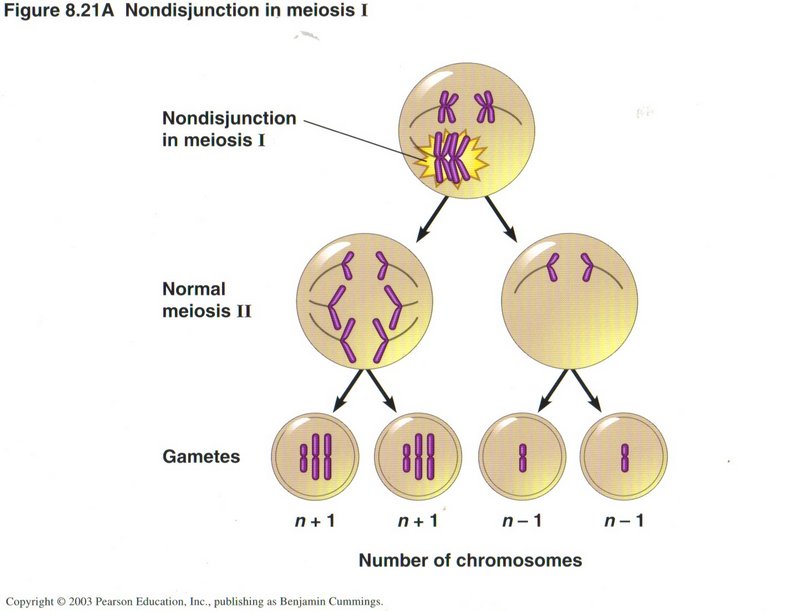
Nondisjunction
A second class of error in meiosis is called nondisjunction. In this error the kinetochore attaches both sets of chromatids to
microtubules going to the same pole and none to a microtubule going to the other pole. As shown in this view for meiosis I, the
result is two gametes each receiving one extra chromosome and the other two gametes each receiving one less. For most types of
human chromosomes, this abnormal number of chromosomes will result in infertility of the gamete or the embryo will be aborted
(miscarried). If human chromosome 21 has an extra chromotid (one out of 700 births), a child inheriting this chromosome suffers
from Down’s syndrome, usually being mentally retarded and having various bodily defects. If a human male has an extra X
chromosome (one out of 2000 births) or a female lacks an X chromosome (one out of 5000 births), the resulting person usually has
lessened characteristics of his or her sex and some characteristics of the opposite sex. A male with an extra Y (one in 2000) or
a female with an extra X (one in 1000) is usually normal, but there may be some effects in either sex. For most other chromosomes,
the error is fatal and the sperm or egg is infertile, or the embroyo or fetus containing the abberated chromosome is miscarried.
Click here for next page
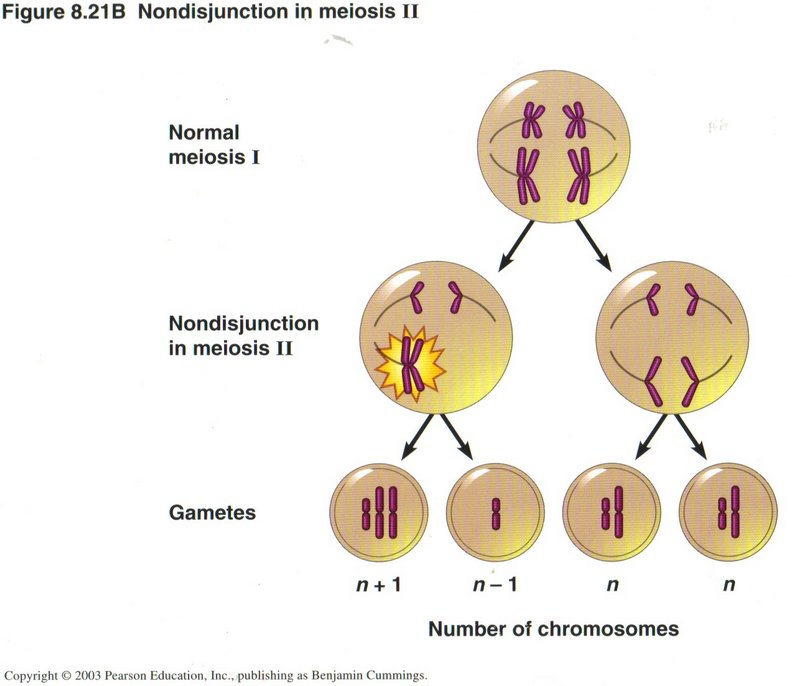
However, if the nondisjunction occurs in meiosis II, as shown here, only half of the gametes are abnormal, as you would expect.
Click here for next topic.
Organic Chemistry ― the Chemistry of the Carbon Atom
As you learned in basic physics, compounds of two or more elements hold together by sharing their outermost rings of electrons (eight
in all elements but hydrogen and helium). Carbon, with four electrons, can combine with many elements, either as having four “extra”
or being “short” four electrons in its outermost shell. In particular, six carbon atoms can form a ring by sharing one electron with
each carbon atom neighbor and sharing the two others with hydrogen, oyxgen, nitrogen, or other carbon structures to make up the
substances of which living things are composed. It can even combine with itself by sharing one electron with each of four neighboring
atoms in one of the two lattices, graphite or diamond. The chemistry of such carbon compounds is so complex that it forms its own
scienctific discipline, called organic chemistry.
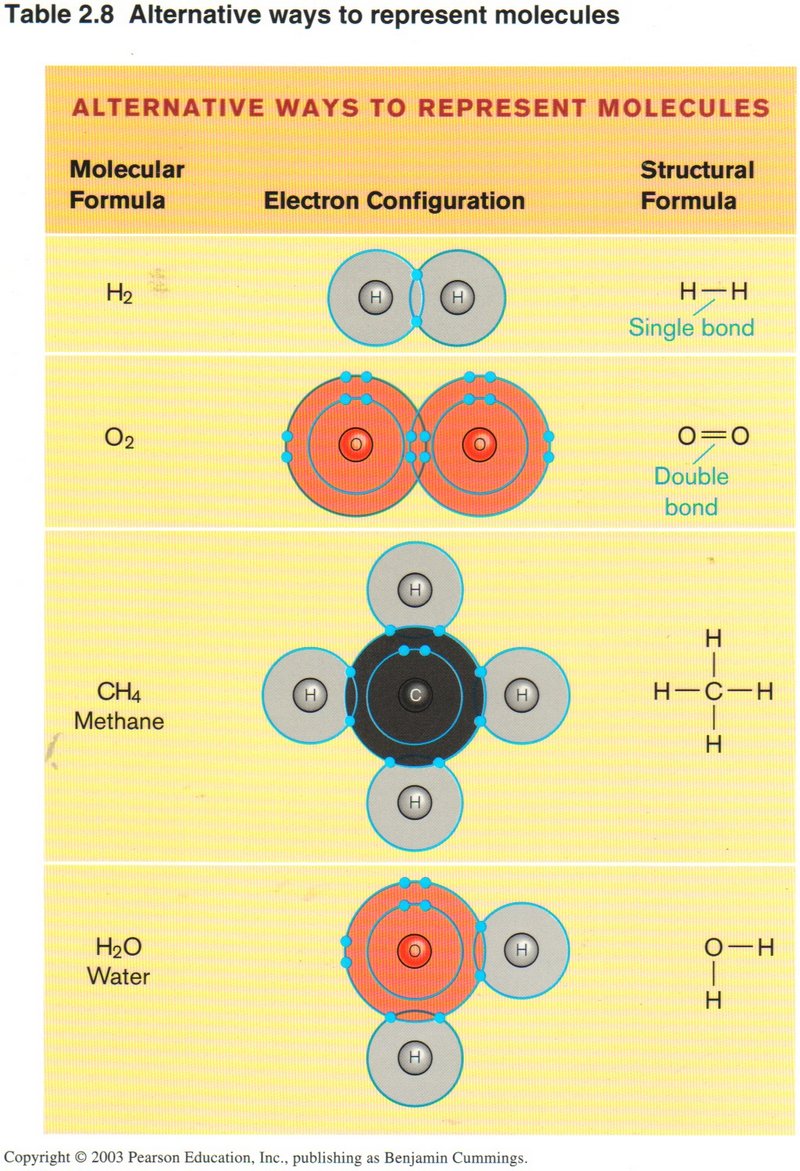
Organic chemists have devised a shorthand way of showing the structure of the complex molecules of carbon and related elements. The
view on the left illustrates this skeleton type of shorthand in the rightmost column for four common substances: Hydrogen
(H2), oxygen (O2), methane (CH4) and water (H2O). Remember that water is polarized ― the
oxygen end of the molecule is negative, due to the outer ring of electrons surrounding the oxygen atom, whereas the opposite end of
the water molecule is positive, due to the fact that the single electron surrounding the positive proton of each hydrogen atom spends
much more time with the oxygen atom (due to its six protons) than with the single proton of the hydrogen atom. Thus substances which
have clusters of hydrogen atoms on the outside of the molecule are repellant to water (hydrophobic), whereas substances that have
other atoms on their exteriors are attracted to water (hydrophyllic).
Click here for next page.
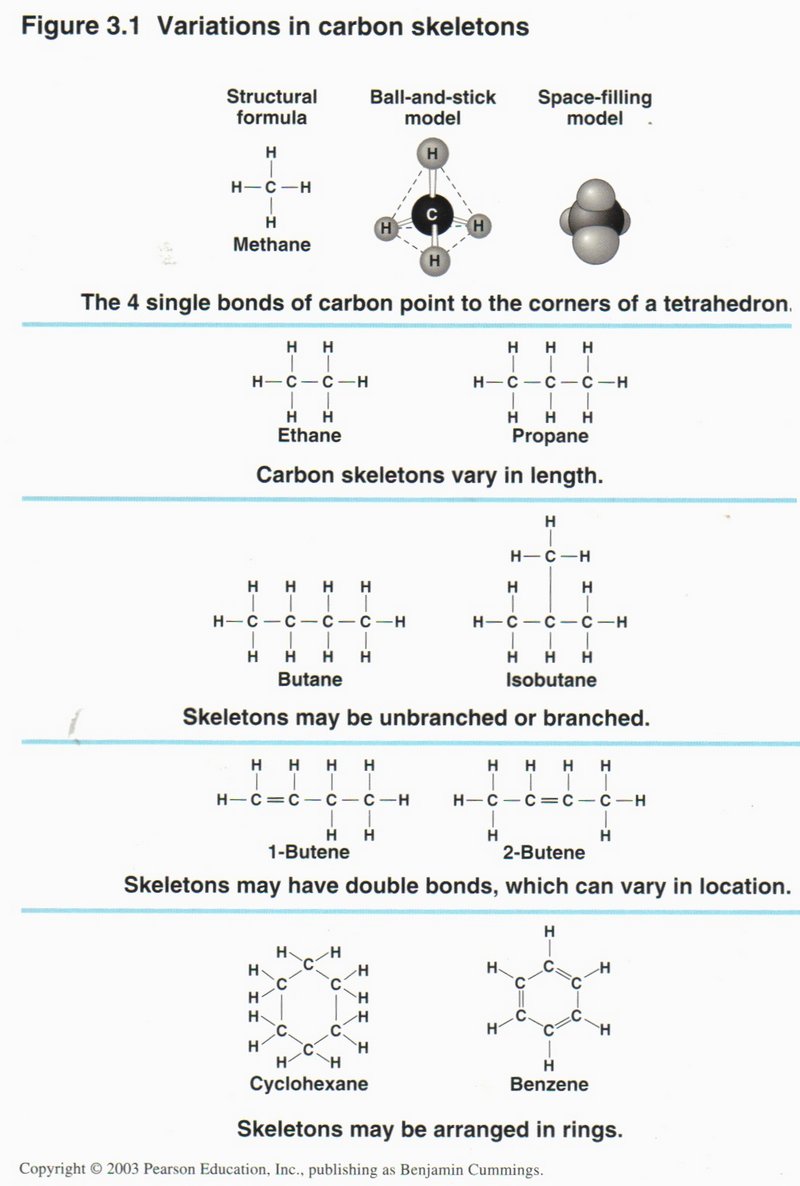
The view on the right applies the skeleton shorthand to some well-known
carbon compounds: ethane, propane, butane, isobutane, 1-butene, 2-butene, cyclohexane, and benzene.
Click here for next page.
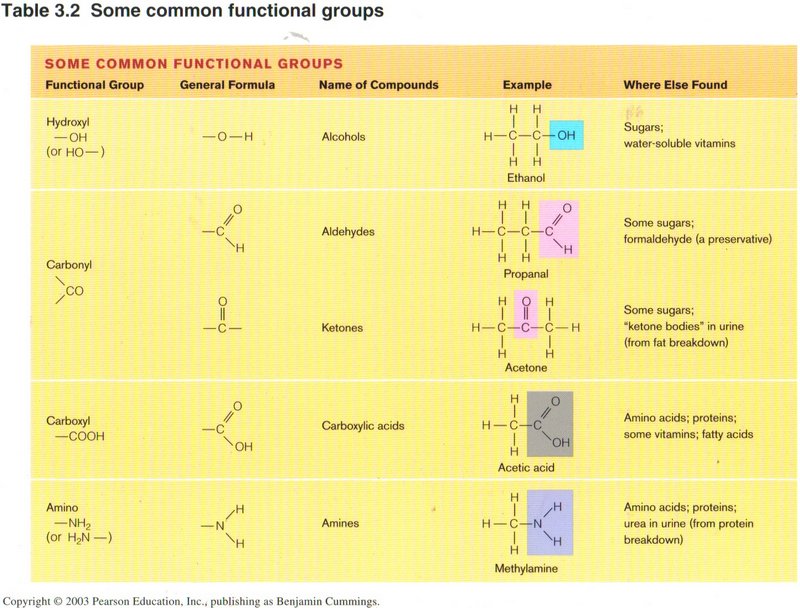
The view above gives illustrations of the shorthand in common compounds containing specific structures of C, 0, H, and N: hydroxyl,
carbonyl, carboxyl, and amino.
Click here for next page.

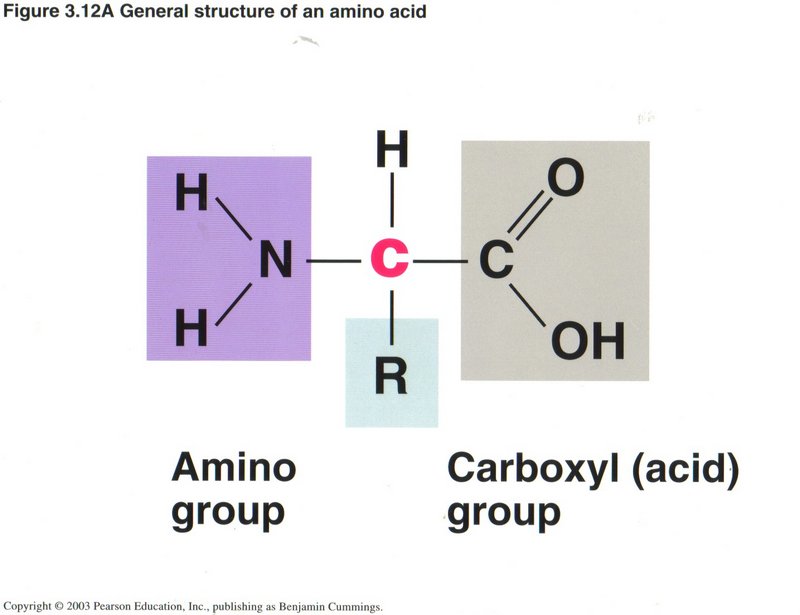
Amino acids
Since amino acids are such vital elements of living cells (particularly as composing all proteins), we present the basics of an amino
acid here. Note the standard structure of an acid on the right, called the carboxyl group, and the standard structure of amine on the
left, called the amino group. The R group varies for the many different kinds of amino acids, but all three groups are always tied
to the pivotal carbon atom shown in red.
Click here for next page.
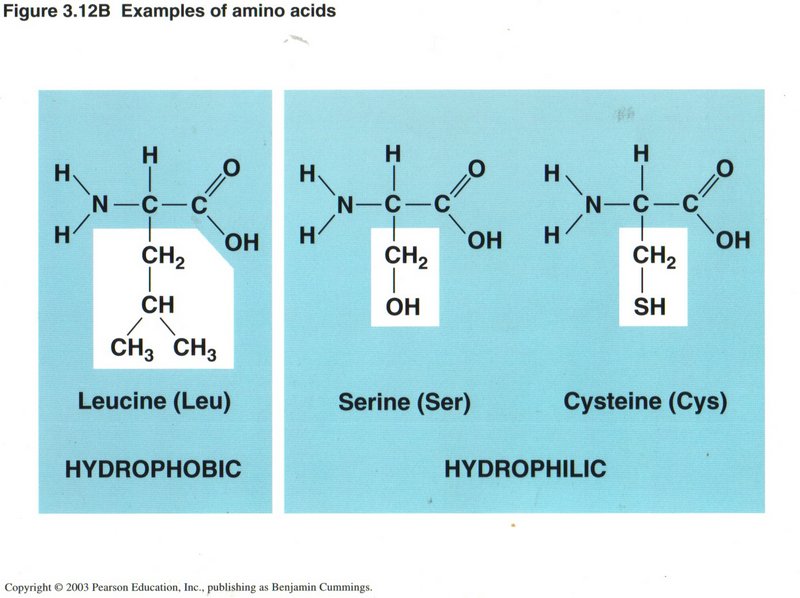
Here we have three different amino acids, all with simple R groups. Those R groups which expose hydrogen atoms are called hydrophobic
(they hate water molecules), whereas those with OH or SH exposed groups are hydrophilic (they love water molecules).
Click here for next page.
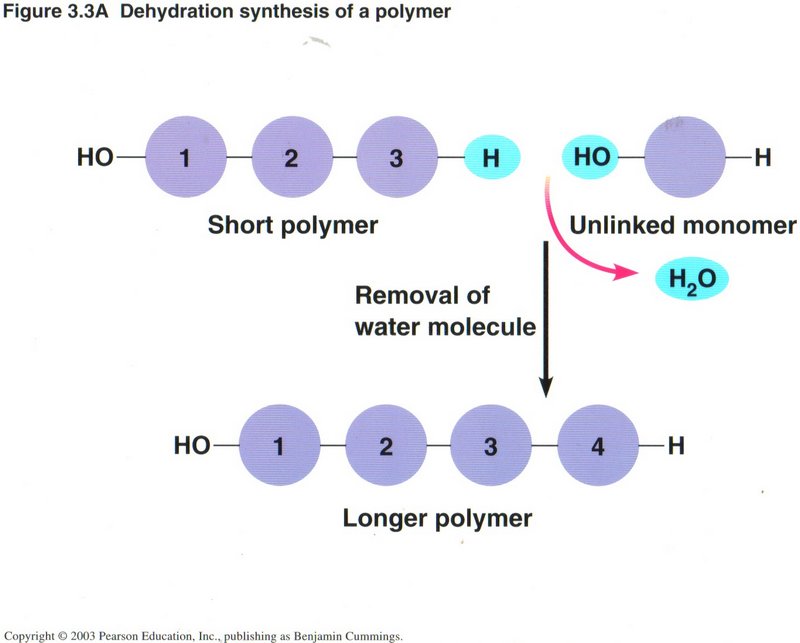
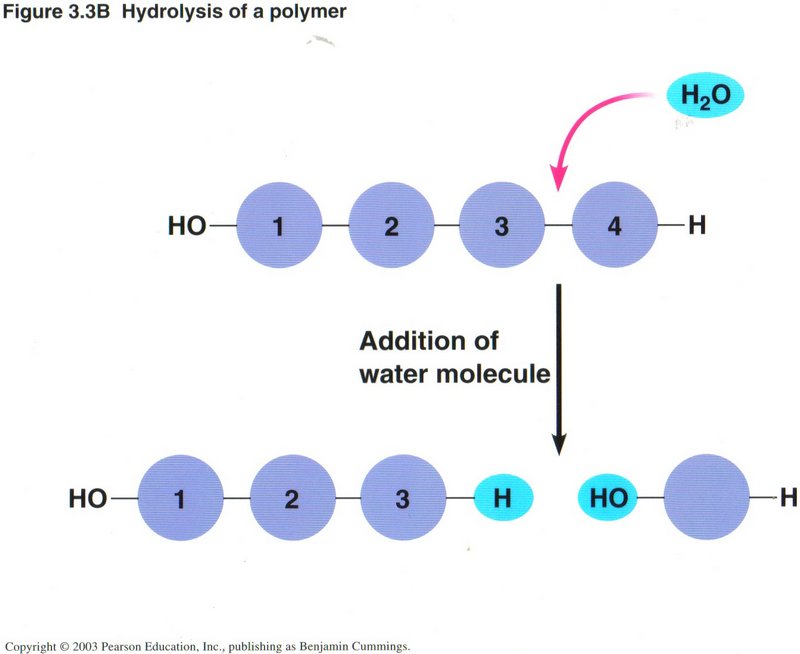
The view on the left illustrates the most common way of constructing a chain of carbon atoms, wherein a monomer (compound with one
carbon atom) is joined to a polymer (compound of several to many carbon atoms) by removing a molecule of water from the two reacting
molecules, a process called dehydration. Conversely, a longer polymer may be shortened by the addition of a molecule of water,
called hydrolysis, as shown in the view on the right. A high percentage of the chemical reactions taking place in living cells
are either hydration or dehydration. Such reactions may require the input of energy, or they may produce energy, depending on the
substances involved.
Click here for next page.
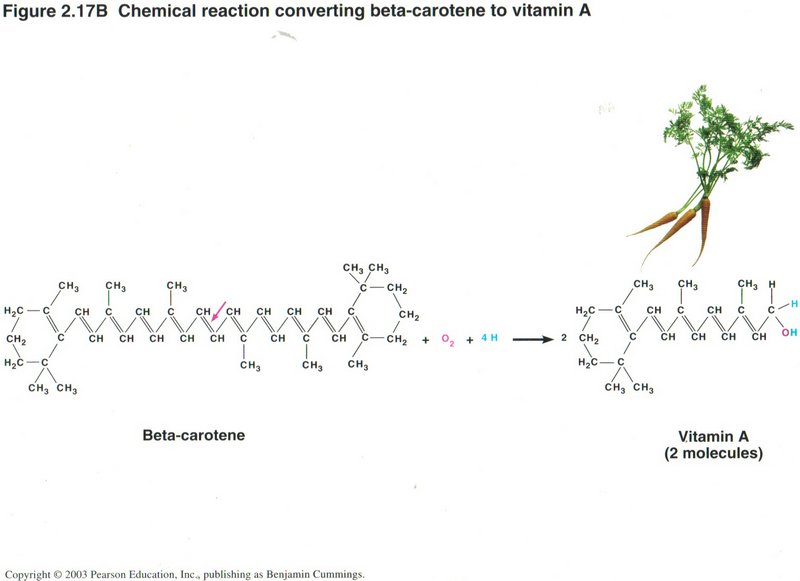
Above we have an example of hydration, breaking down a complex molecule like beta-carotene into two molecules of vitamin A.
Click here for next page.
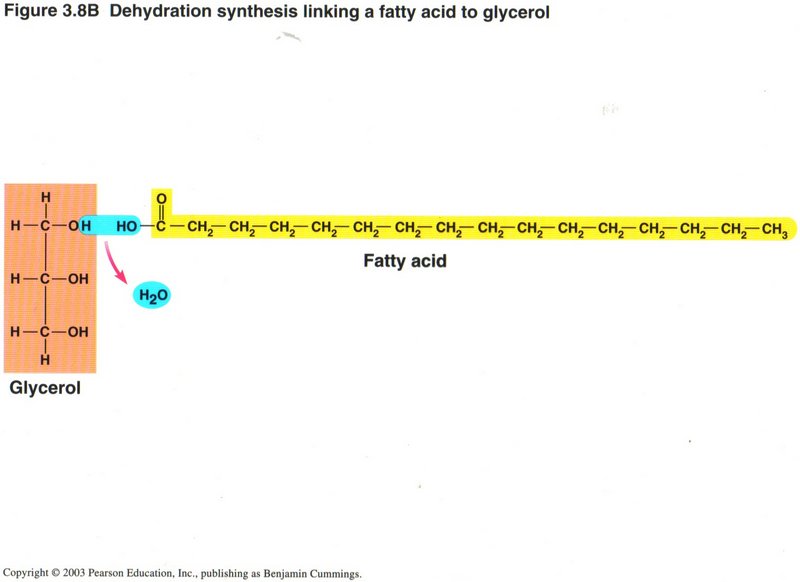
Above is an example of dehydration creating a more compolex molecule: Linking a fatty acid to glycerol.
Click here for next page.
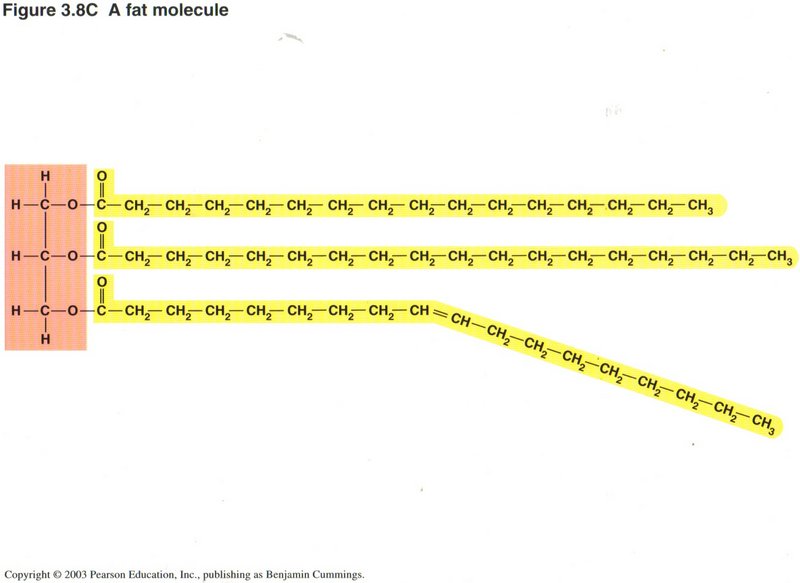
Here we repeat the process of the preceding illustration two more times to form a fat molecule. Note that while the first two chains
are totally composed of CH units (called saturated), the third chain has a double bond in the center, caused by deleting two hydrogen
atoms from successive carbon atoms (unsaturated). Such sites are prone to be unstable, both chemically and physically (may change
orientation).
Click here for next page.
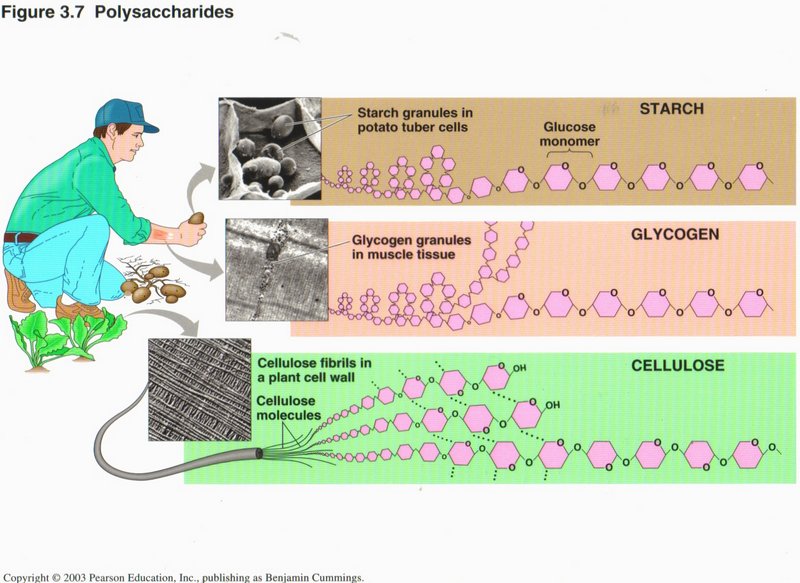
Here we see the complex nature of the molecular structure of some common substances, wherein the carbon and hydrogen atoms are not
explicitly shown, but other atoms (like oxygen) are: starch, glycogen, and cellulose.
Click here for next topic.

In the view at left we see the structure of the eukaryotic (true nucleus) type of cell found in all advanced plants and animals; it
is obviously extremely complex. We will first examine the cell as a whole and then discuss the individual organelles.
In this typical animal cell, the nucleus dominates the cell and contains the principal part of the DNA. It is in the nucleus
that the DNA is duplicated, a process called replication. It is also in the nucleus
that the generation of RNAs takes place in the process called transcription. There are several forms of
these. Messenger RNA (mRNA) is the ribbon from which proteins will be made in the ribosomes ― those little red dots
peppering the surfaces of the nuclear envelope and the rough ER (rough endoplasmic reticulum) ― in the process called
translation. Ribosomes are formed from another type of RNA ― rRNA (ribosonal RNA) ― plus several complex proteins. A
third type of RNA ― tRNA (transfer RNA) finds the specific amino acids needed to compose the protein (originally called a
polypeptide)and brings them to the ribosome. The rough ER finishes the processing of the proto-protein by chemically
altering some of its amino acid constituents and usually by folding it into its final shape. The rough ER also manufactures a number
of chemical molecules needed by the cell, like membrane units and enclosed vehicles, called vesicles, for carrying specific
substances. The smooth ER (smooth endoplasmic reticulum) manufactures fats and carbohydrates, including the sugars, needed to
synthesize DNA and RNA. We will discuss the Golgi apparatus, the cytoskeleton, the plasma membrane and the
mitochondria, in detail below.
Click here for next page
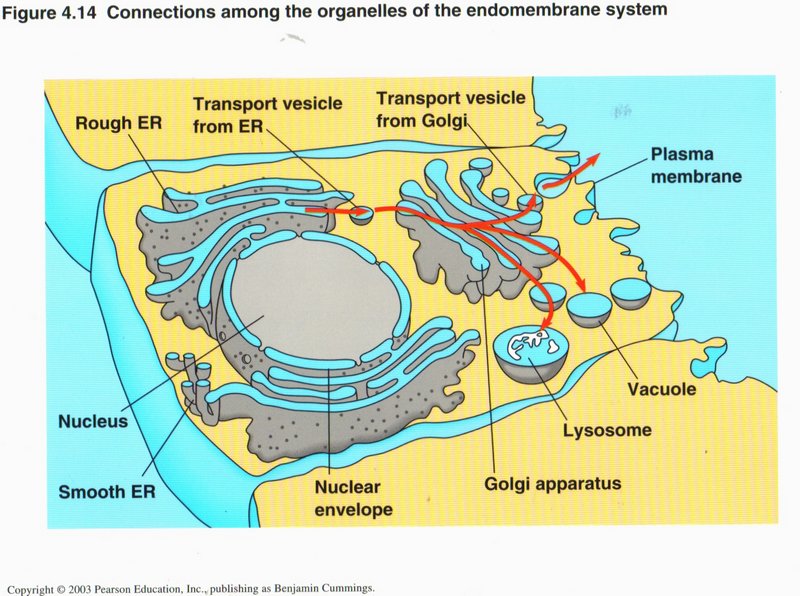
I. Organelles of a Eukaryotic Cell
Here we see the major system of membranes that carry out most of the cell’s chemical activities, as well as send and receive vesicles
enclosing proteins and other substances. Whereas the rough and smooth ERs are connected to each other and to the nucleus, the Golgi
apparatus is separate, being concerned primarily with vesicles, vacuoles, and lysosomes. We will examine each organelle in turn.
Click here for next page
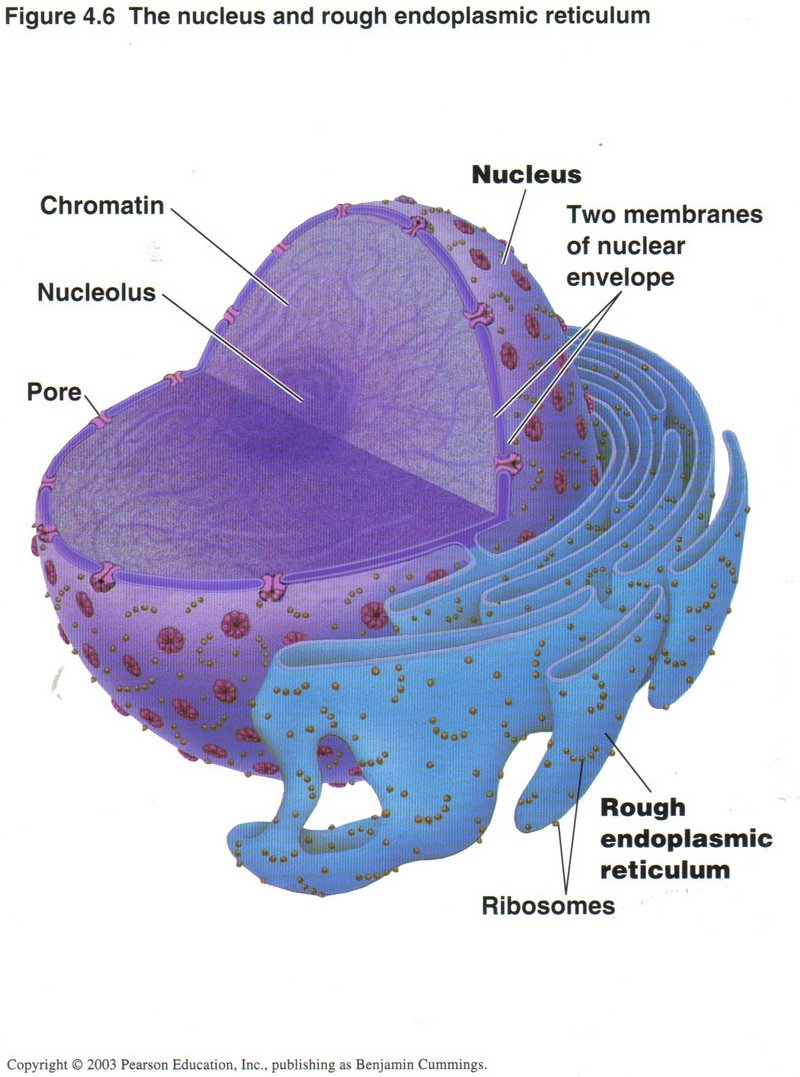
The nucleus and rough endoplasmic reticulum (ER)
The nucleus is where the principal set of DNA molecules reside (called the genome) which occur in several different modes.
In this view the mode is chromatin, where the individual genes of the DNA molecules are accessible to the specialized proteins
controlling transcription. Many such sets are at work simultaneously, generating long ribbons of mRNA, which are transported
through the pores of the nuclear membrane to ribosomes, mostly on the surface of the rough ER membranes, as shown. The
ribosomes use the mRNA as templates to synthesize polypeptides, which pass into the rough ER for chemical modification and
folding, where they become proto-proteins.
Click here for next page
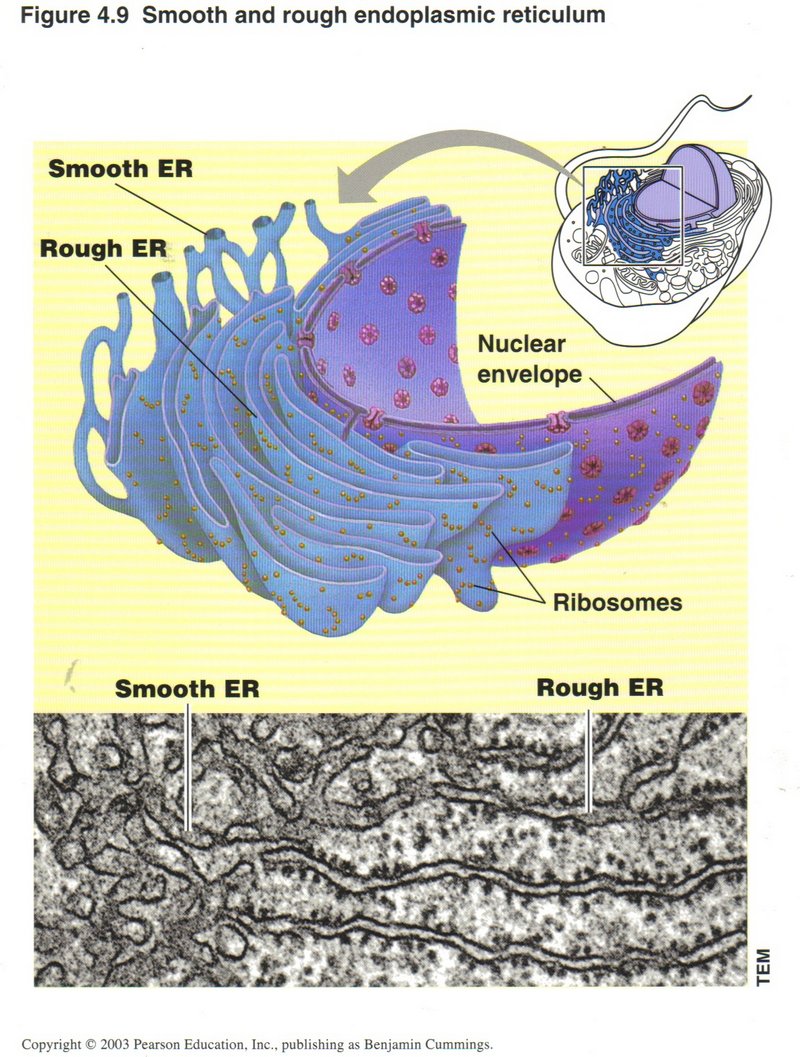
Rough ER (Endoplasmic reticulum)
These two sets of folded membranes are where most of the chemical modification of proteins takes place (rough ER) and where various
substances needed by the cell are manufactured (in both rough and smooth ERs).
Click here for next page
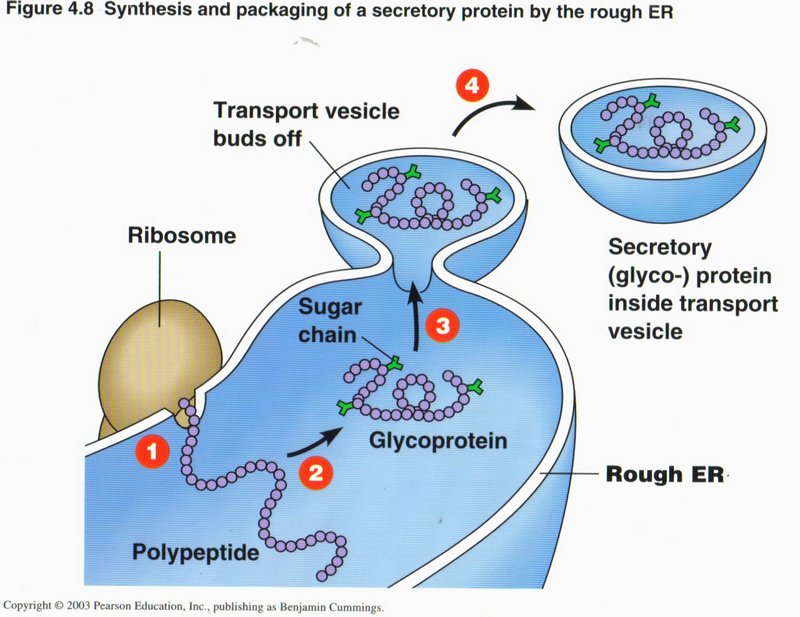
In this view we see the sequence of events that completes the preparation of a protein. (1) The proto-protein emerges from the attached
ribosome where it has been synthesized from the mRNA ribbon transcribed from a gene in the DNA of the nucleus (mRNA ribbon not shown).
(2) This particular protein requires an attachment of a sugar molecule to several places on the protein (shown in green). Other
modifications and/or folding may take place as well. (3) The location where the protein molecule is completed is made into a vesicle
by the pinching off of a portion of the rough ER membrane. (4) The vesicle separates from the rough ER and is taken to its destination
in the cell by sliding along a microtubule (not shown). By being in a vesicle the protein and its associated sugar appendages are
protected from destructive enzymes that are usually present in the cell cytoplasm.
Click here for next page
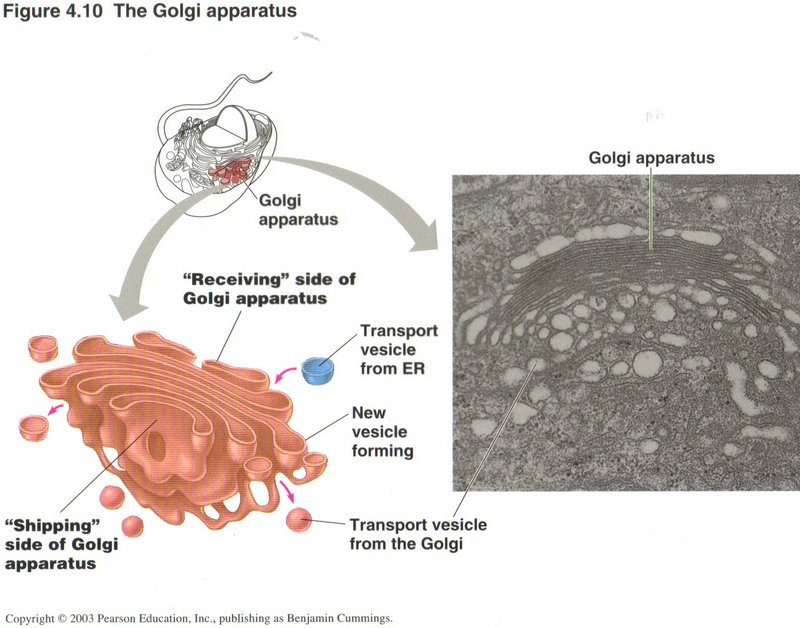
The Golgi Apparatus
The Golgi apparatus acts as a central storage and routing facility for the transport vesicles that carry substances between sources
and destinations within the cell. It has modification roles on some proteins, and can sense and modify the caps on the transported
molecules to ascertain their treatment and/or destinations. It is here that lysosomes (see below) are produced and launched
as vesicles.
Click here for next page.
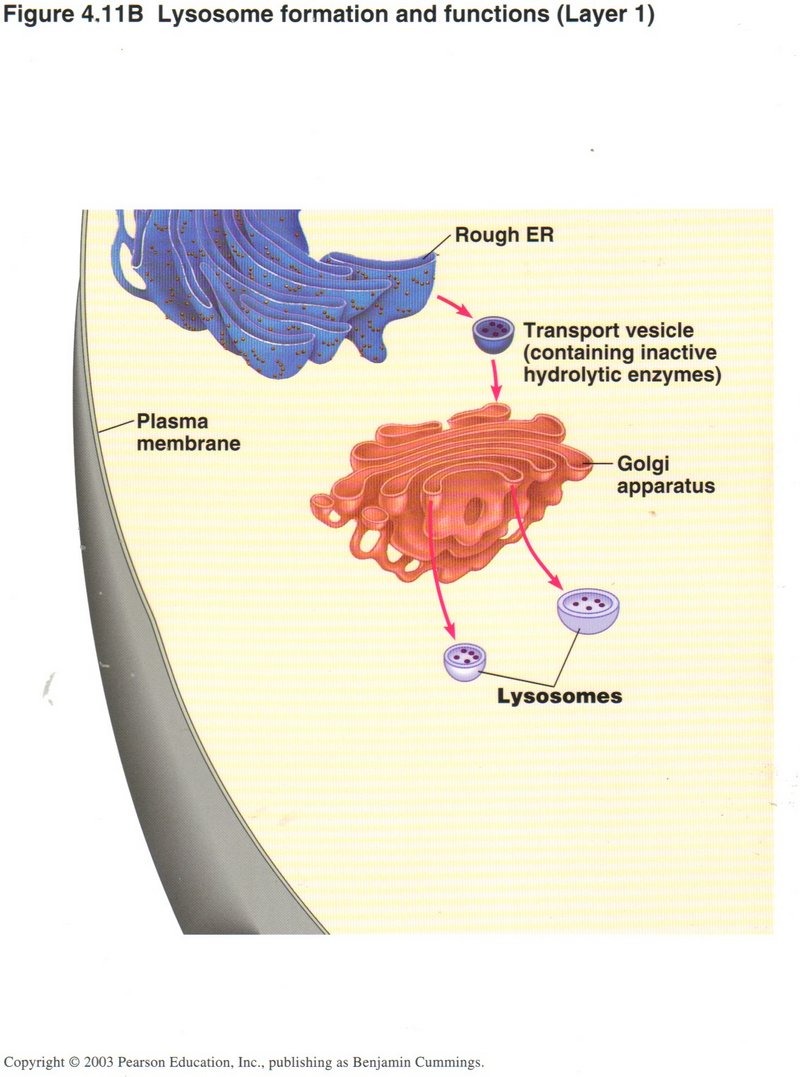
Lysosomes
In this first view, a transport vesicle generated in the rough ER containing inactive hydrolytic enzymes (type of protein) arrives at
the Golgi apparatus. Here these enzymes are activated by chemical modification and launched as lysosomes.
Click here for next page.
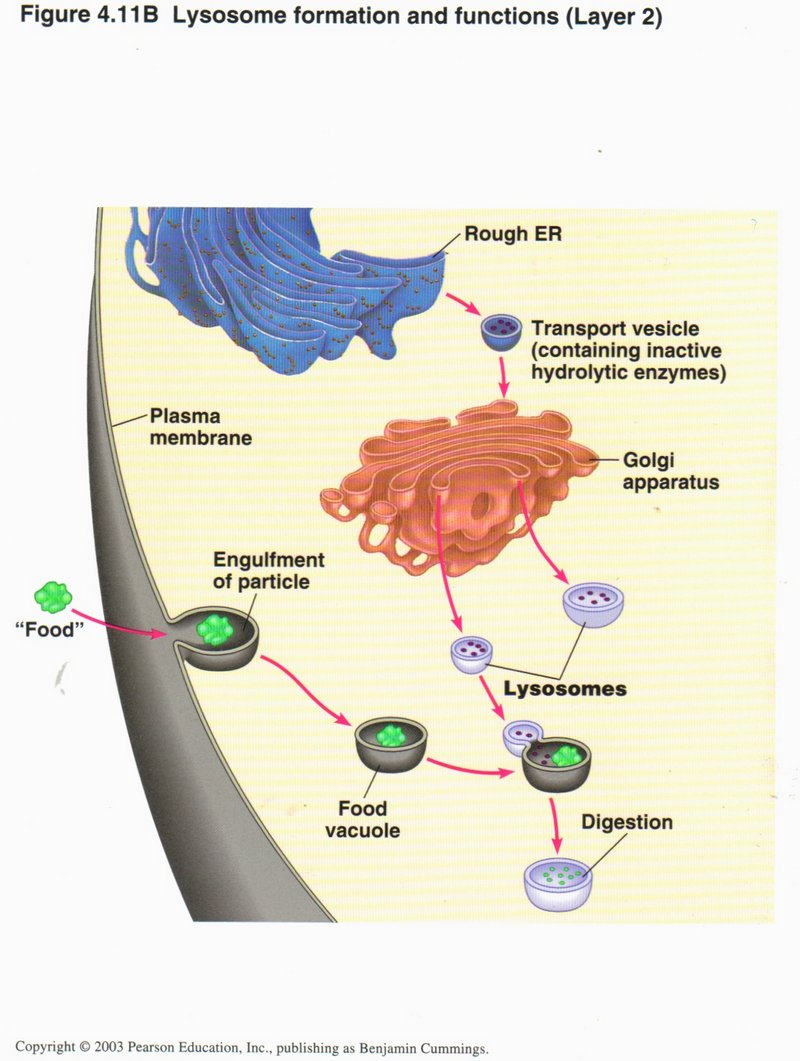
Meanwhile some food for the cell has arrived at the plasma membrane (left), enclosed in a vesicle (called a food vacuole) and encounters
the lysosome, which fuses with the vacuole, allowing the enzyme to digest the food -- break it down into simpler molecules that will
later be synthesized into DNA or RNA bases or meet other cell needs.
Click here for next page.
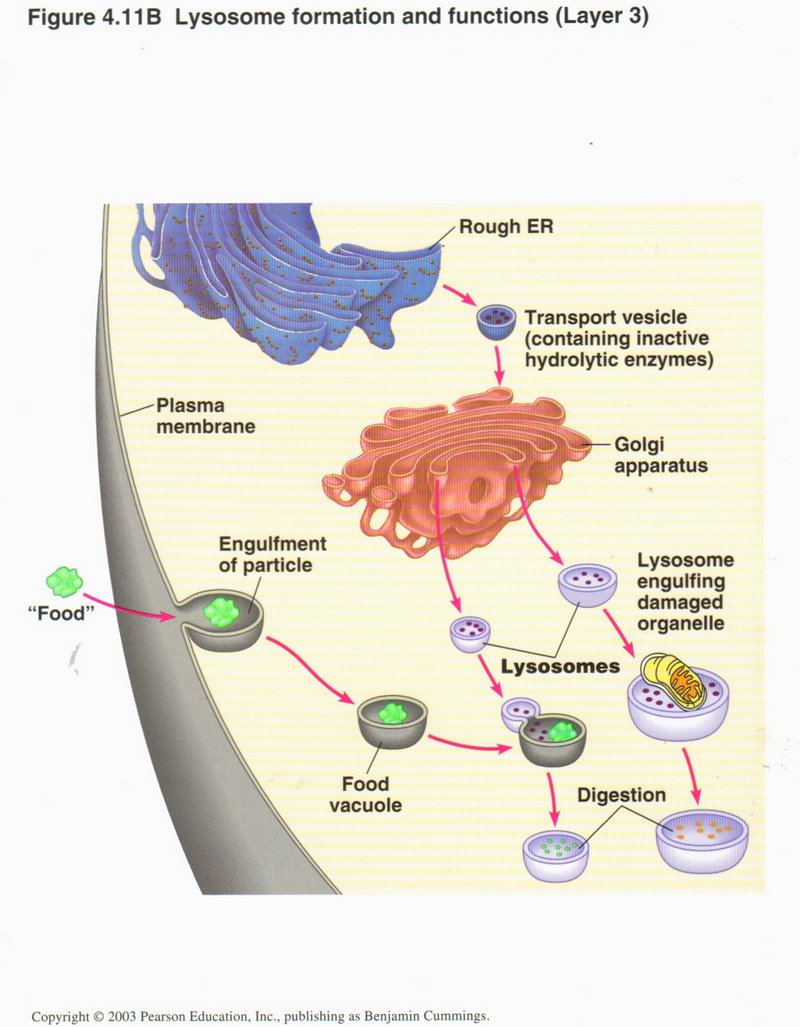
The lysosome may also engulf a damaged organelle (a mitochondrion here) and reduce it to simple molecules by digestion.
Click here for next page
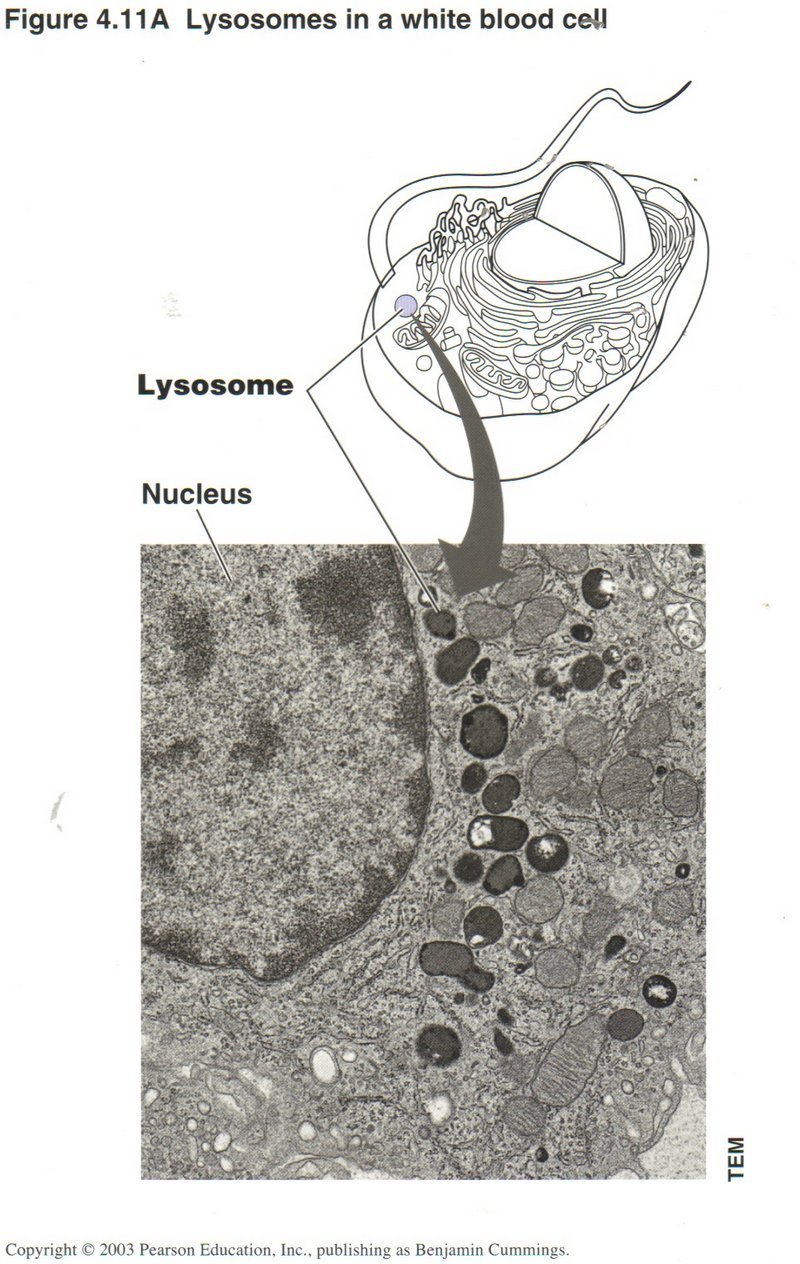
In this view are shown the representation and actual microphotographs of lysosomes in a white blood cell, in which scavenging action
by the destructive enzymes in the lysosome are a principal function.
Click here for next page

Cytoskeleton
There are three types of fibers which are found in the cytoskeleton (see left bottom of cell structure): The microfilament, composed of actin (protein) subunits,
about 7 nanometers across; the intermediate filament, composed of twisted fibers, and about 10 nanometers across; and the
microtubule, from its composition of a two-unit protein complex called a tubulin subunit, about 25 nanometers across.
The microfilament and microtubules are dynamic, as they are often growing at one end and decomposing at the other. The intermediate
filament is more stable.
Click here for next page
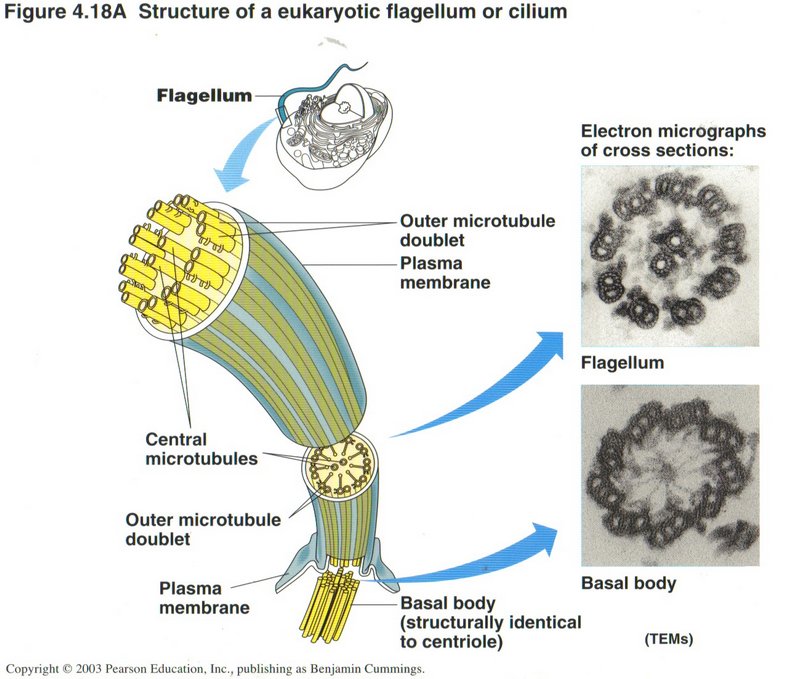
Here we see the complex structure of a flagellum, the whip-like tail that propels a cell through its liquid medium. Only certain
types of cells have these appendages.
Click here for next page
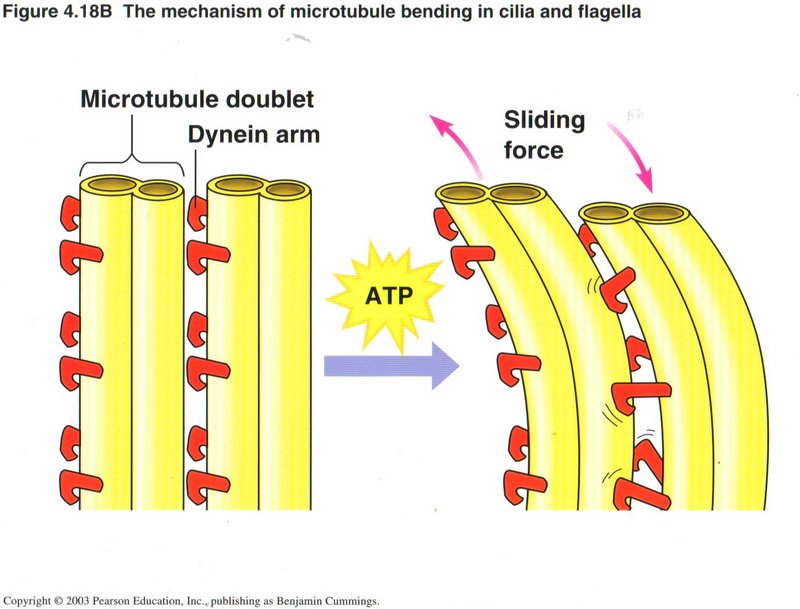
Here we see how motion is created within the cell. Dynein arms on the tubule act as ratchets on a neighboring tubule to propel
the first tubule along the second, using a molecule of ATP to power each ratchet movement. This is the basic method of creating
muscular movements and also for moving vesicles or organelles within the cell from one place to another.
Click here for next page
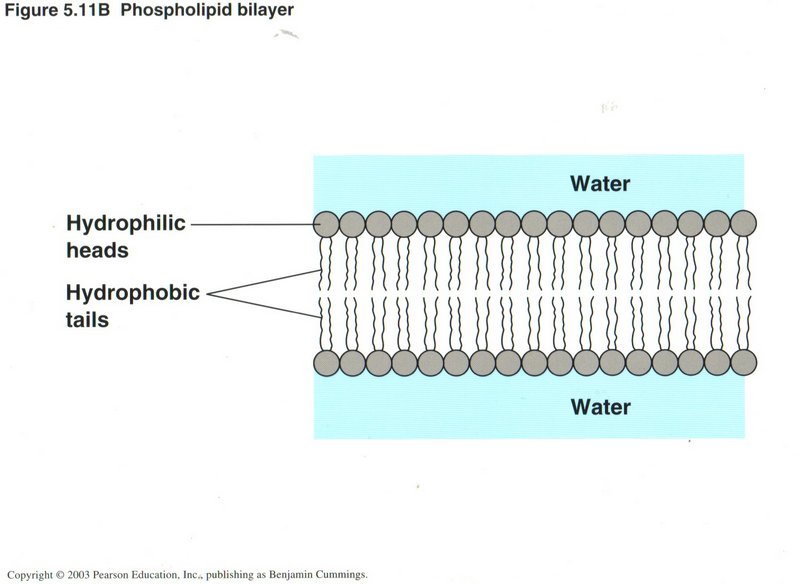
Membranes
There are numberous membranes in the cell, including the cellular envelope, the plasma membrane. Most are constructed of
two layers of phospholipid molecules, where the phospho part (round heads) are hydrophilic (water tolerant) and the fatty-acid tails
(lipids) are hydrophobic (water-repellant). The pressure of the water on the two sides of the membrane keep its two layers tightly
pressed together.
Click here for next page
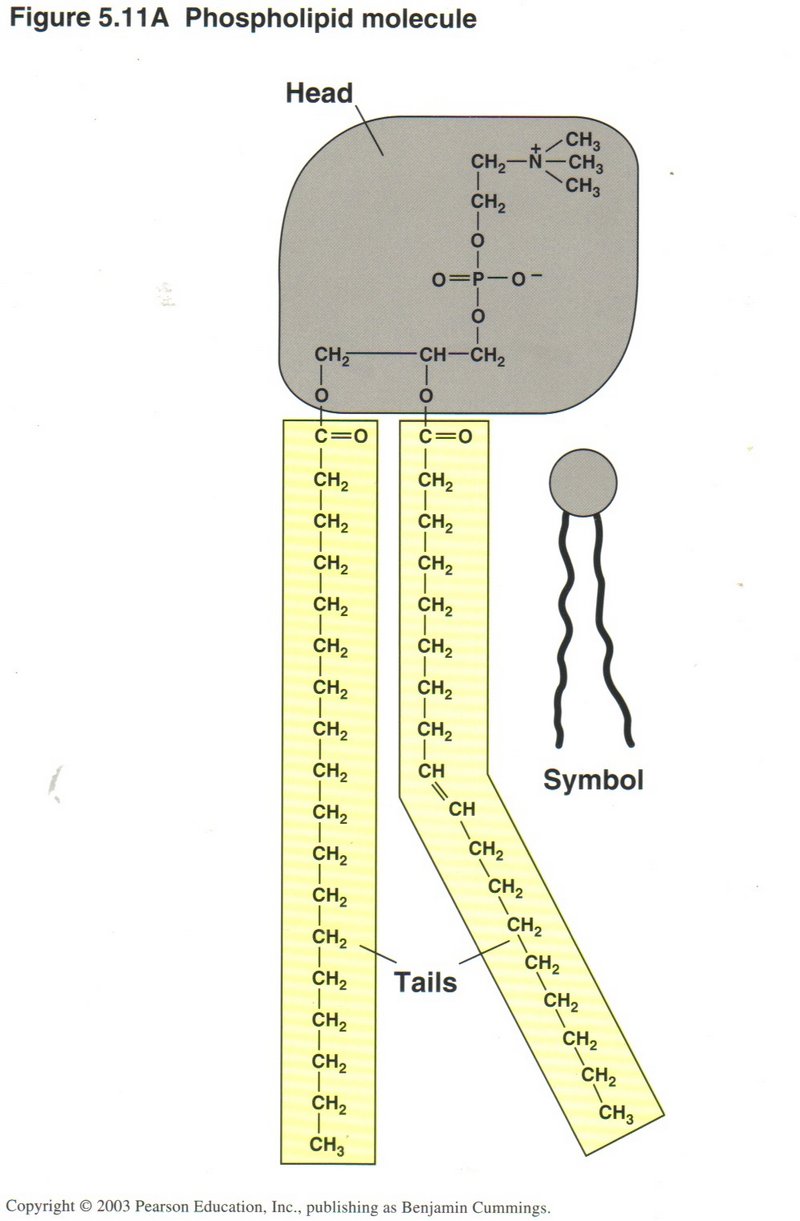
This view shows the chemical compostion of the phospholipid molecule. As shown, the tails may be straight or angled, enabling the
molecules to cling together in the membrane.
Click here for next page

Here you can see something of the complexity of the plasma membrane surrounding the cell. Studded throughout the membrane are proteins
of many kinds. Some provide recognition sites on the external side of the membrane for specific substances in the extracellular medium.
Some bind specialized messenger proteins (called hormones) that trigger many different functions in the cell’s life cycle. Some
provide pores which permit selected substances to enter or leave the cell. And some are involved with moving ions (H+,
Na+, Ca2+) (hydrogen, sodium, calcium positive ions) into or out of the cell.
Click here for next page
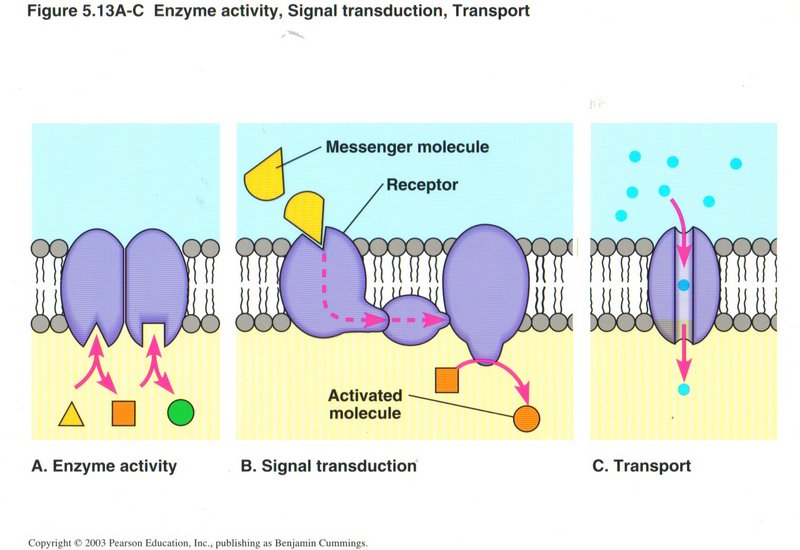
As this view shows, most membranes have embedded within them numerous protein complexes, three types of which are shown: (a) enzyme
activity, either enabling or inhibiting various enzymes; (b) signal transduction, wherein an external messenger molecule is received
by the receptor on the exterior of the membrane, causing the protein complex to change shape, and activate a protein on the inside of
the membrane; and (c) provision of pathways for ions and other substsances to enter or leave the cell.
Click here for next page
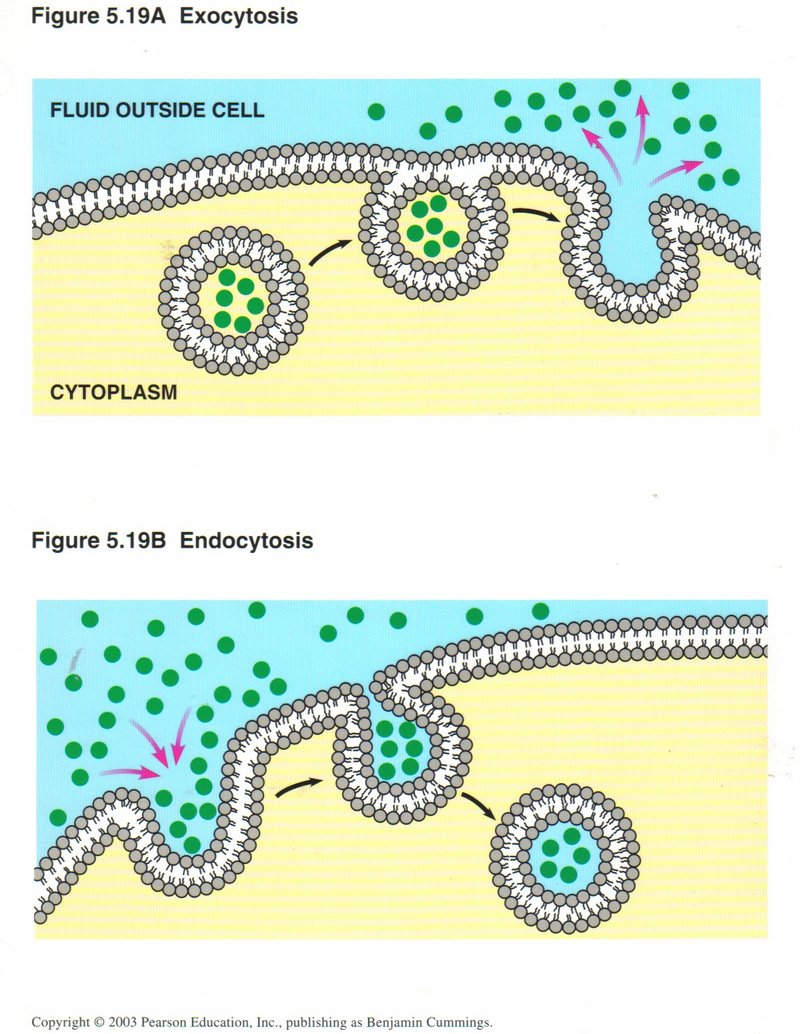
The upper view shows the process of exocytosis ― how the membrane accepts vesicles containing substances to be ejected from the
interior of the membrane, fuses the vesicle into its structure, and deposits the contents of the vesicle on the outside of the
membrane The opposite process ― endocytosis (lower view) ― takes substances from the outside of the membrane by invagination,
forming a vesicle from its structure which encloses the admitted substances and releases the vesicle into the interior of the membrane.
Thus the membrane is constantly changing its circumference, but never breaks the insulation it provides between the inside and outside
of the cell or organelle.
Click here for next page
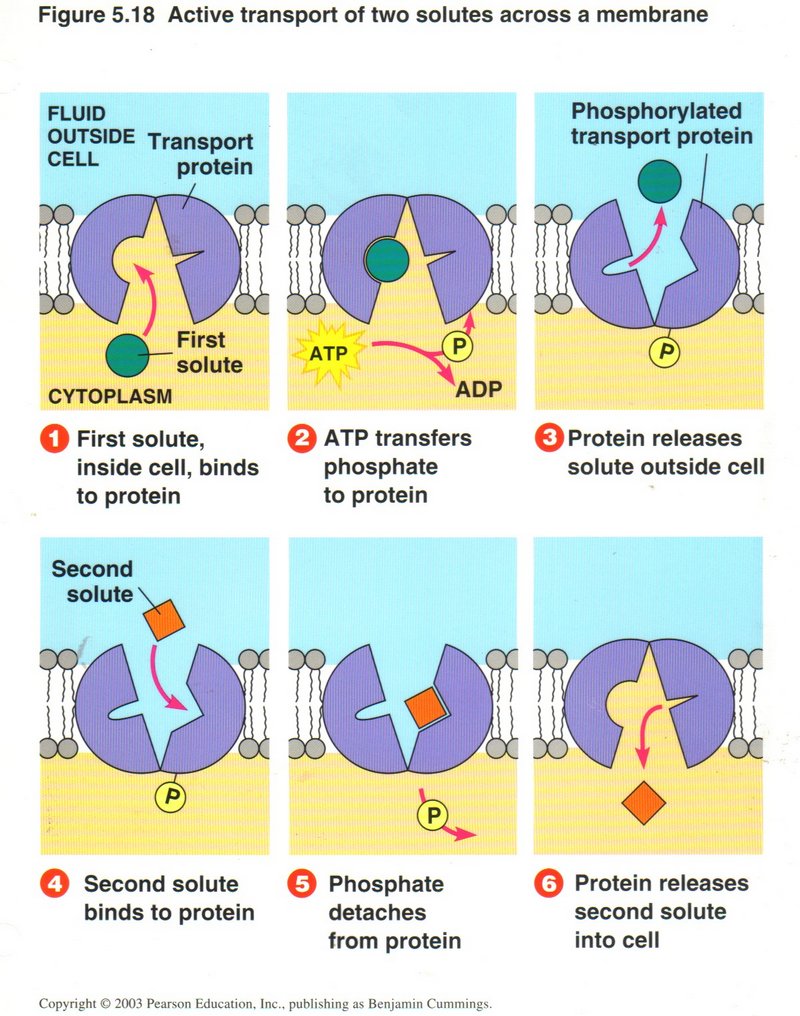
In this view, the transfer of one substance from inside to outside is counter to the concentration of that substance, and requires
energy to accomplish. Here the protein is shown changing its shape on being energized by ATP to send the first substance across the
membrane from inside to outside. The release of the ejected substance allows the protein to return to its original shape, and in so
doing admit another substance into the cell or organelle.
Click here for next page
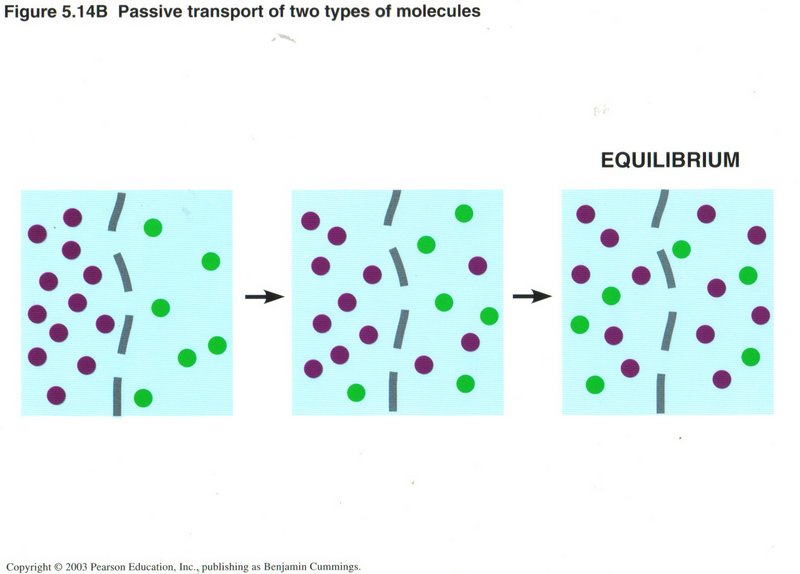
 Some membranes in the cell are permeable (one the molecules can transit). Molecules in solution migrate throughout the domain
available to them. If a higher concentration exists on one side of a permeable membrane, they will quickly move through the membrane
until the concentrations on the two sides are equal. No energy is needed.
Some membranes in the cell are permeable (one the molecules can transit). Molecules in solution migrate throughout the domain
available to them. If a higher concentration exists on one side of a permeable membrane, they will quickly move through the membrane
until the concentrations on the two sides are equal. No energy is needed.
This ability to equalize concentrations on the two sides of a permeable membrane is true for any number of solute ions or molecules,
as shown here for two.
Click here for next page
Mitochondria
The mitochondria are the powerhouses of the eukaryotic cell. They contain the machinery which transforms the energy of food
products (fats, sugars and starches) into the energy of the ATP (adenosine triphosphate) molecule, which is the universal
source of energy for all cells, as well as a component of DNA and RNA. There are two processes that perform this transformation:
glycolysis and the citric acid cycle plus chemiosmosis. Glycolysis, performed in both bacteria and higher
forms of life, generate two ATP from the basic food unit (glucose). Cells containing mitochondria generate as much as 38 ATP per
molecule of glucose, a tremendous increase in available energy. A mitochondrion has its own DNA, with all the genes and
protein-producing machinery found in the nucleus, which leads some biologists to believe it may have arisen from a bacterium.
This seems to me highly doubtful, since bacteria do not contain mitochondria. In order to replicate, mitochondria are dependent upon
several vital proteins produced only from the nucleus.
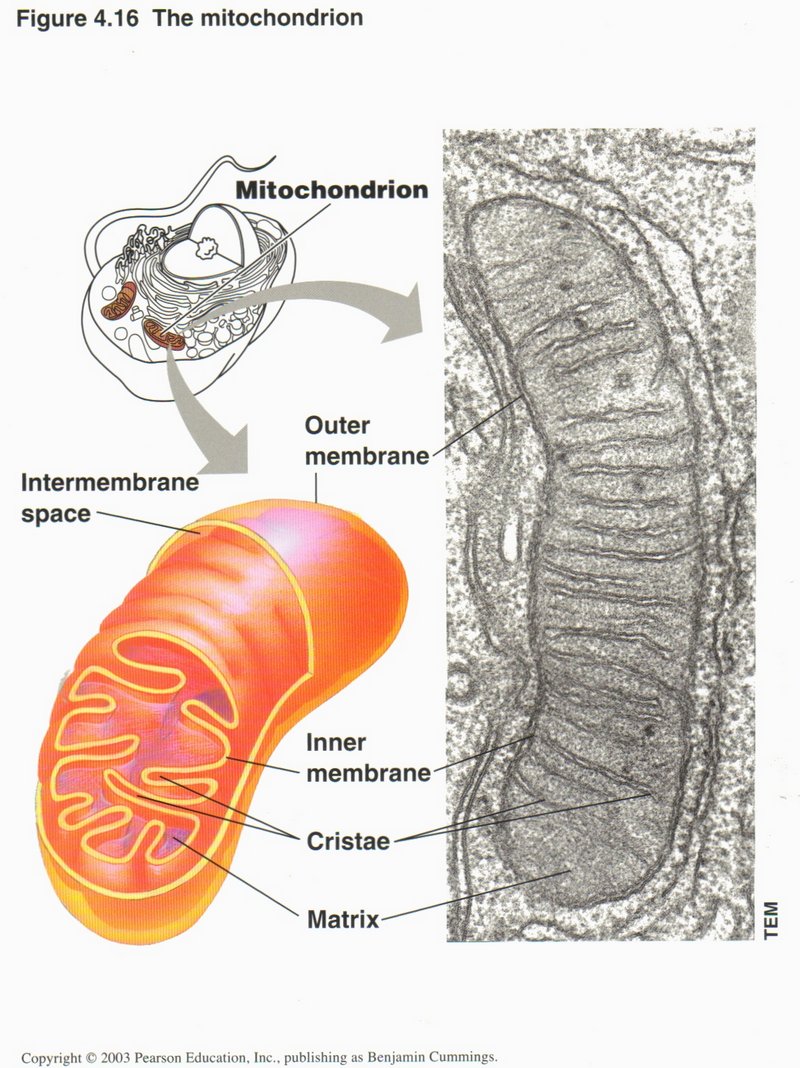
Here we see the structure of a mitochondrion. The outer membrane is similar to the outer membrane of the cell itself, and the
inner membrane to the rough ER. The matrix within the inner membrane contains DNA, replication proteins and proteins for generating
the energy molecules ATP (adenosine triphosphate), NADH (nicotinamide adenine dinucleotide), and FADH2
(flavin adenine dinucleotide). These are the principal energy sources used in all processes of the cell requiring energy. The chemical
reactions are quite complex and are catalyzed by more than 10 different protein complexes. Thus the mitochondrion is truly the
powerhouse of the cell. Since it can reproduce itself, there are from 10 to several hundred in the average cell, with some egg cells
of birds and reptiles having hundreds of thousands of copies. They do not occur in bacteria, but some think they originated
therefrom.
Click here for next page or Click here for a description of energy development in the cell.
Summary of cell functions
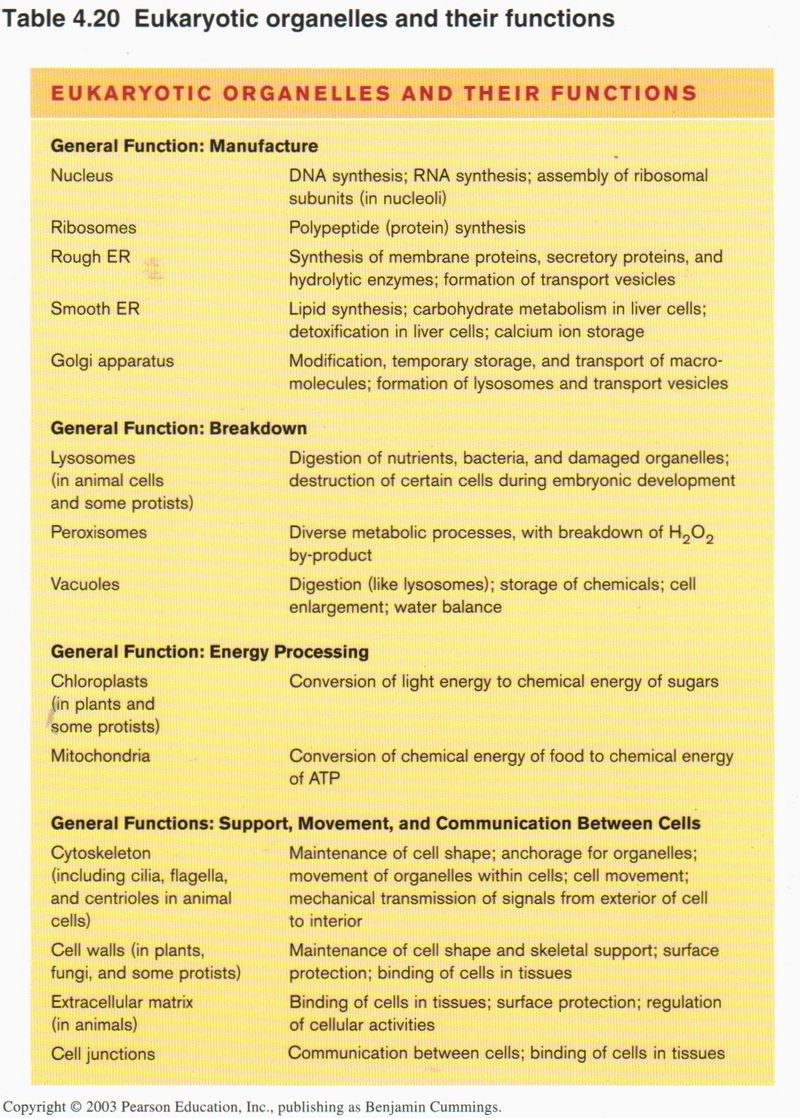
This table summarizes the many functions carried out by the several organelles in the cell. Most of these functions are carried out
in the bacterial cell as well, although that type of cell has no interior membranes.
Click here to return to next topic.
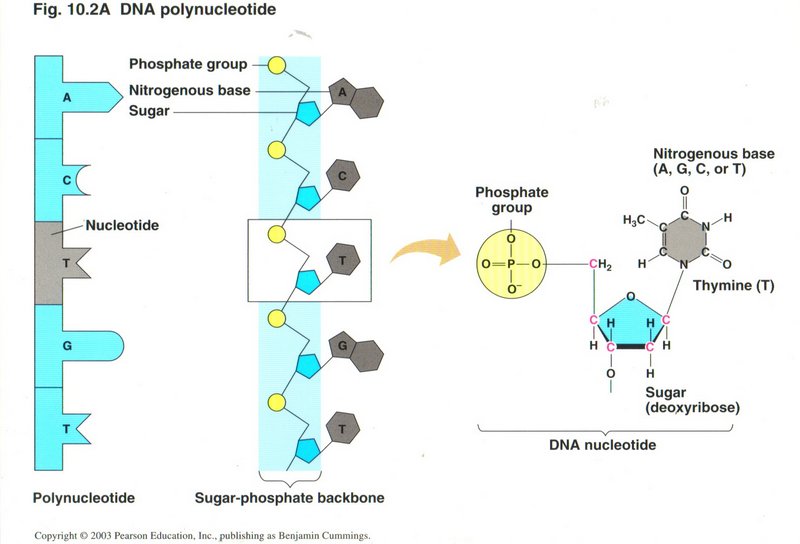
This view shows a single string of nucleotides in the leftmost column, and the same string as they are held in the phospho-sugar ladder in the
center column. The highlighted nucleotide (T - thymine) is shown in diagrammatic detail on the right.
Click here for next page.
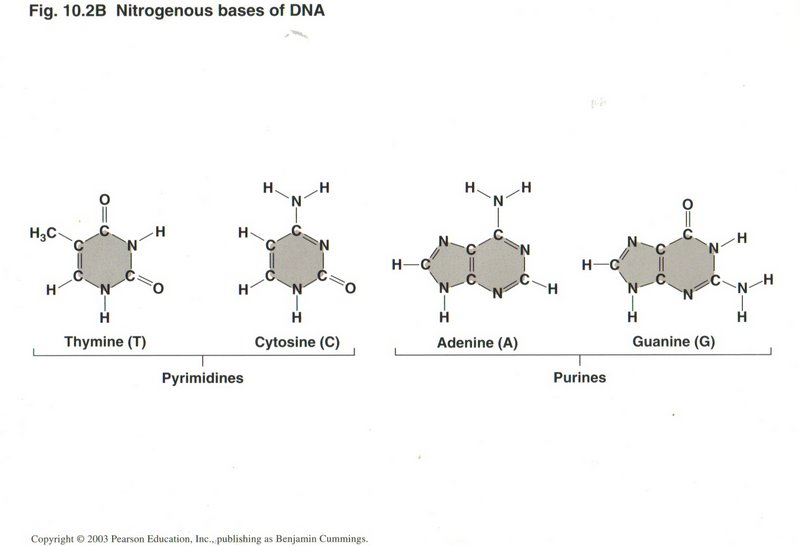
Here we see all four of the nitrogenous bases that constitute the rungs of the DNA ladder: Thymine ("T"), Cytosine ("C"), Adonine ("A"), and
Guanine ("G"). They are always paired C-G or A-T, because that pairing maximizes the hydrogen bonds between the pair, considerably
stronger than any other possible pairing. This single fact permits nearly perfect replication of the DNA molecule.
Click here for next page.
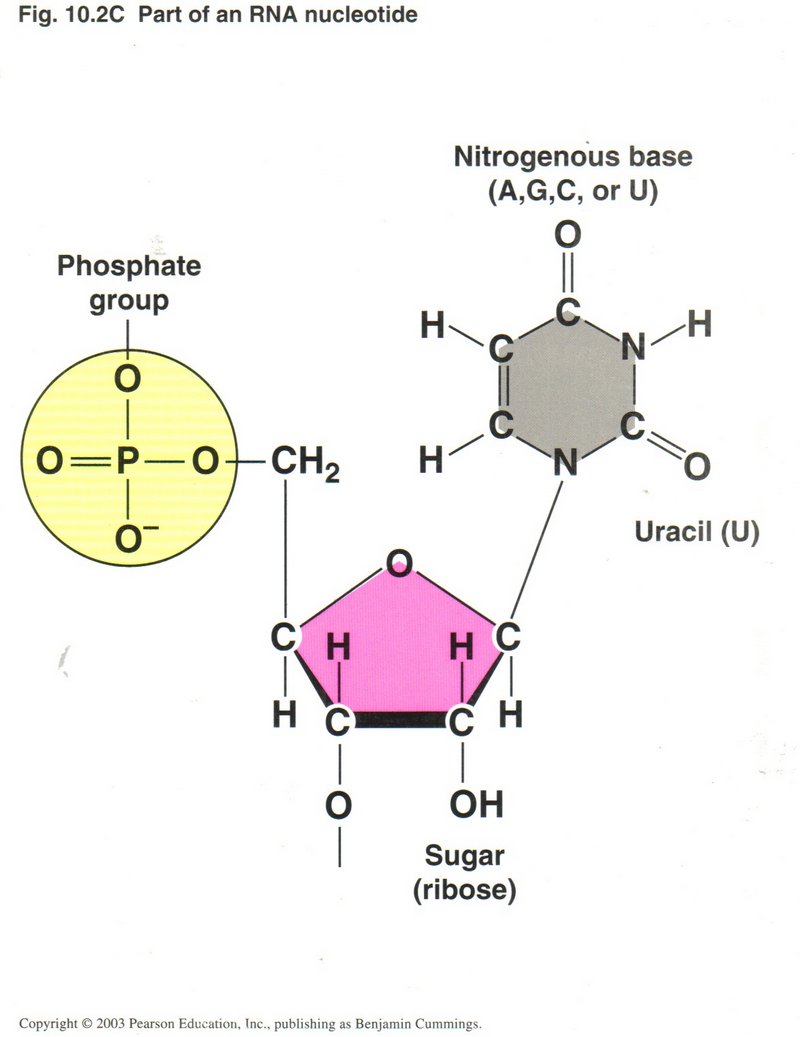
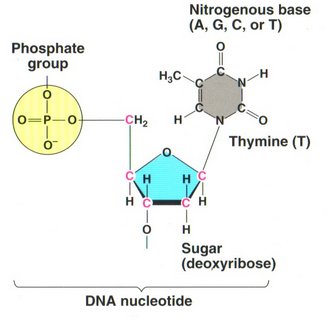 The view at the left shows the structure of the RNA nucleotide (uracil), and that on the right the structure of the DNA nucleotide
thymine. The only two variations are the presence of an OH group on the 2' carbon atom, making the sugar "ribose" in RNA rather than
the sugar "deoxyribose" in DNA (hence the first letter in the acronym DNA or RNA); and the substitution of uracil (U), which has a
single H atom on one of the carbon atoms in the ring rather than H3C on that atom in thymine. All the other bases in RNA
are the same as in DNA. However, these apparently minor differences make a major difference in the behavior of the two molecules.
Whereas RNA is stable in fairly long single strings of base-pairs, DNA is unstable in its single-string form for even short strings.
On the other hand RNA is not stable in a double helix form as is DNA (although some viruses make a short hybrid helix, one-half RNA,
the other half DNA), nor can it attain the enormous lengths that DNA usually does.
The view at the left shows the structure of the RNA nucleotide (uracil), and that on the right the structure of the DNA nucleotide
thymine. The only two variations are the presence of an OH group on the 2' carbon atom, making the sugar "ribose" in RNA rather than
the sugar "deoxyribose" in DNA (hence the first letter in the acronym DNA or RNA); and the substitution of uracil (U), which has a
single H atom on one of the carbon atoms in the ring rather than H3C on that atom in thymine. All the other bases in RNA
are the same as in DNA. However, these apparently minor differences make a major difference in the behavior of the two molecules.
Whereas RNA is stable in fairly long single strings of base-pairs, DNA is unstable in its single-string form for even short strings.
On the other hand RNA is not stable in a double helix form as is DNA (although some viruses make a short hybrid helix, one-half RNA,
the other half DNA), nor can it attain the enormous lengths that DNA usually does.
Click here to return to next topic.
Energy Development in the Cell
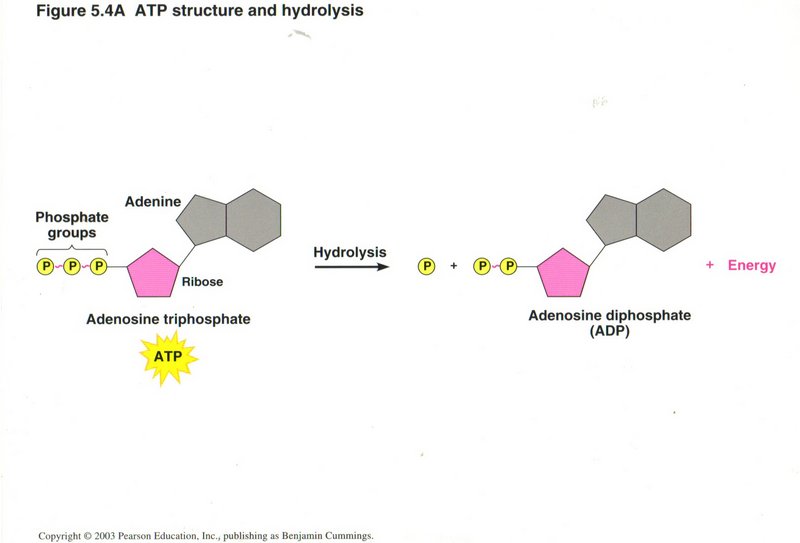
Generation of ATP
Since virtually all the cellular processes requiring energy use ATP as its source, the cell must in turn generation large amounts of
ATP. In this view, we see the structure of ATP and how it produces energy when hydrolyzed to ADP.
Click here for next page
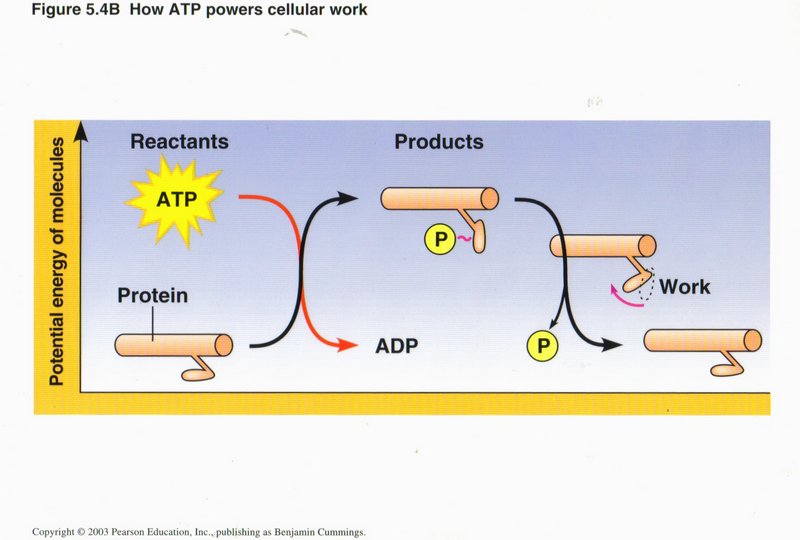
The hydrolysis of ATP to ADP above is an example of an exergonic reaction, in which the energy level of the reactants is greater than
the energy level of the products, the difference in energy becoming available to power other chemical reactions, produce motion, change
the conformation of proteins, or other types of cellular work. In this view, motion is being created, i.e., to move an organelle or
other entity along a microtubule.
Click here for next page
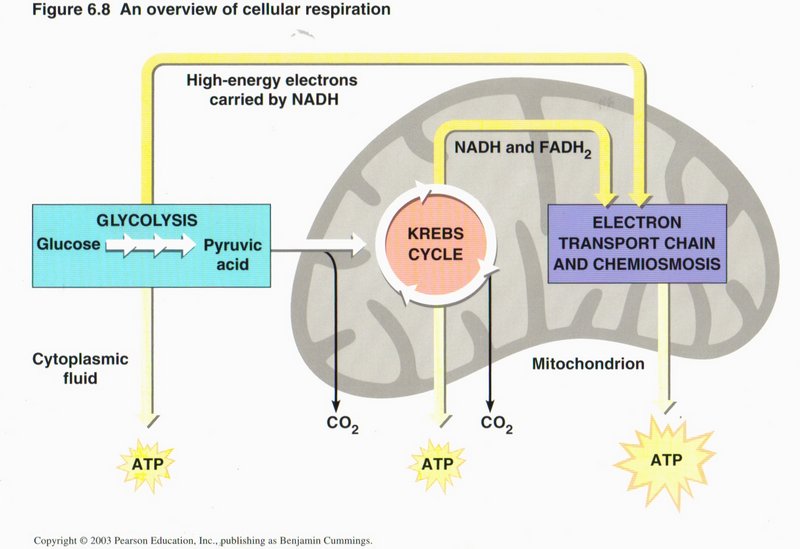
Cellular respiration
Respiration is the name given to the group of processes than combine oxygen with food products and give off CO2. Here
we see three principal methods of so doing: glycolysis (conversion of glucose to pyruvic acid in the cytoplasm), the citric
acid cycle (also called the Krebs cycle), and chemiosmosis, the latter two taking place in the mitochondrion.
Click here for next page

Glycolysis
Here we see the overview of the generation of energy from the breakdown of one molecule of glucose (a simple sugar) into two molecules
of pyruvic acid. This process (called glycolysis) converts two molecules of ADP to ATP and two ions of NAD+ to NADH plus two
H+.
Glucose is a 6-carbon sugar, such as plants produce by photosynthesis, using the energy of sunlight.
Click here for next page
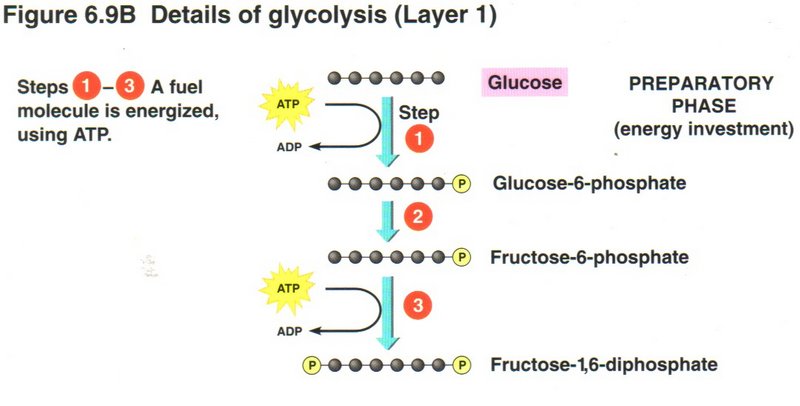
In the first three steps of the process, the cell invests two molecules of ATP to raise the energy level of the glucose to that of
fructose-l,6-diphosphate. Here we see an example of capitalism in the cell’s economy.
Click here for next page
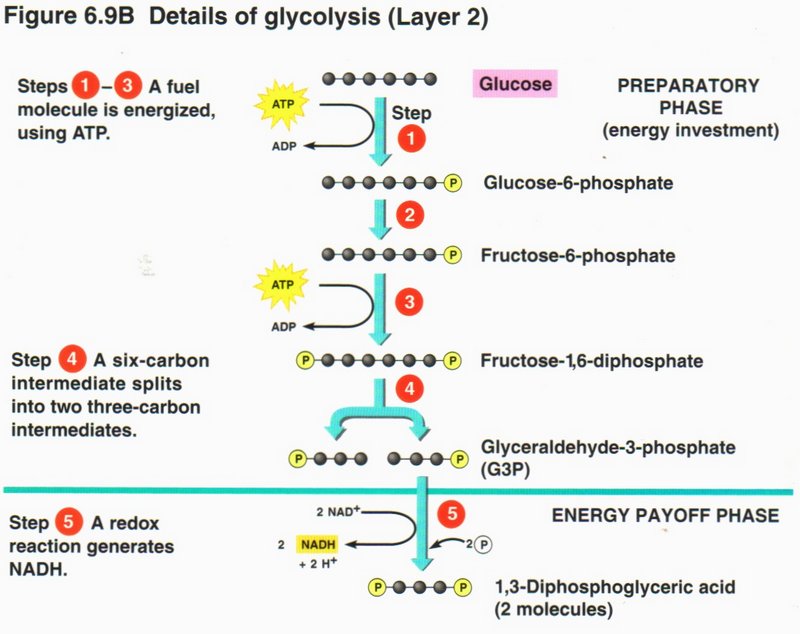
In step 4, an enzyme breaks down the 6-carbon molecule to two 3-carbon molecules (G3P). In step 5, a redox (reduction-oxidation)
reaction upgrades two NAD+ ions to two NADH plus two H+, while converting the two G3Ps into diphosphates.
Click here for next page
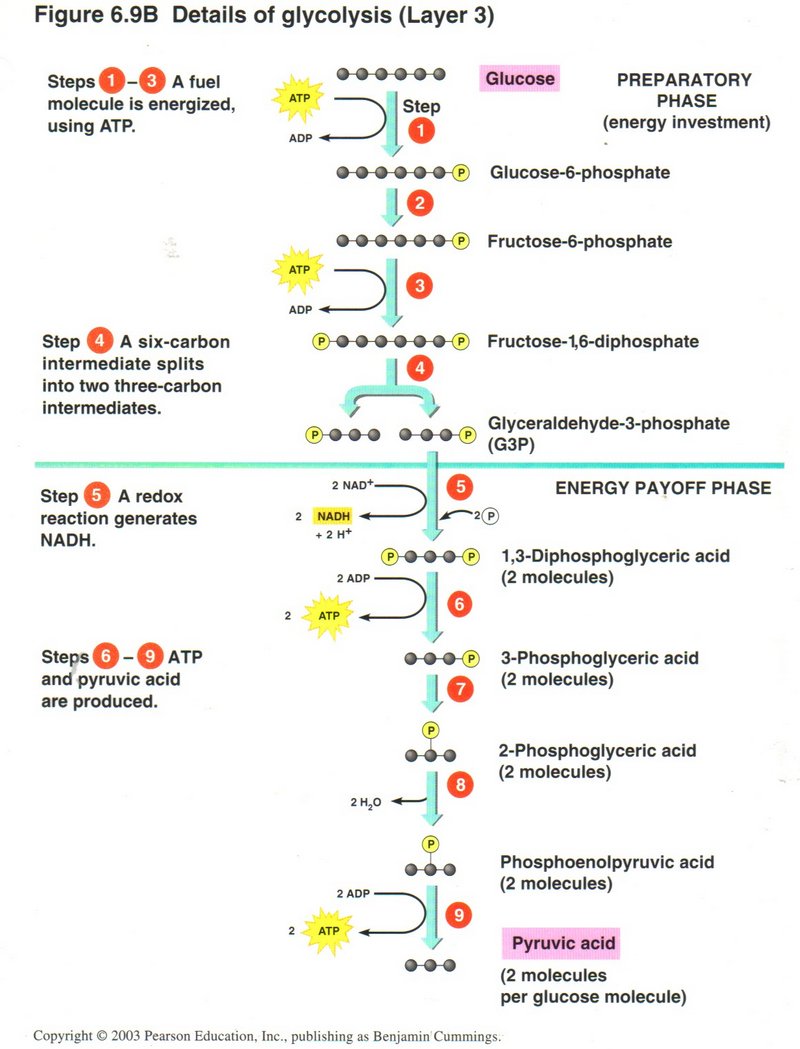
In the next four steps, the four phosphate radicals are successively removed from the two diphosphates and transferred to ADP,
generating a total of four ATP molecules, and yielding two molecules of 3-carbon pyruvic acid as the final breakdown product of the
one molecule of glucose. In the total process, a net gain of two molecules of ADP and two of NADH is accomplished.
Click here for next page
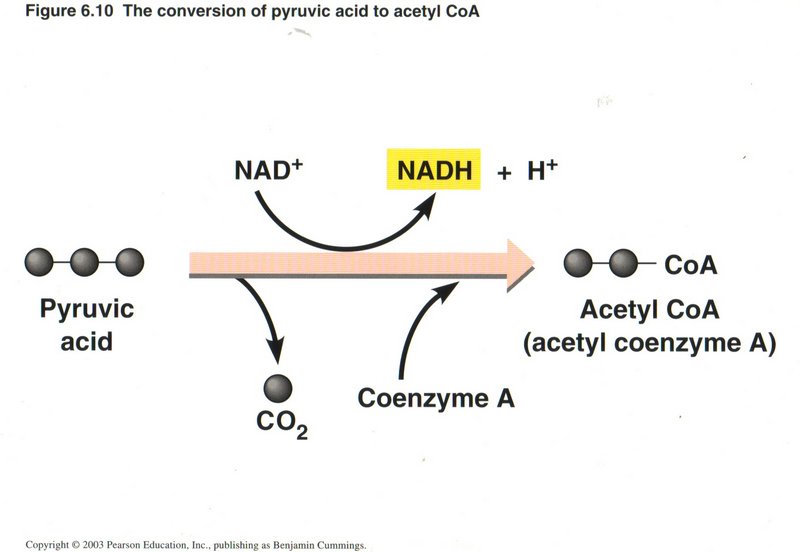
The Citric Acid (Krebs) Cycle
A preliminary step in preparation for the actual cycle of events is the reduction of the 3-carbon pyruvic acid to the complex 2-carbon
(acetyl) radical and coemzyme A, plus a molecule of CO2 This process upgrades another molecule of NAD+ to NADH
per molecule of pyruvic acid.
Click here for next page
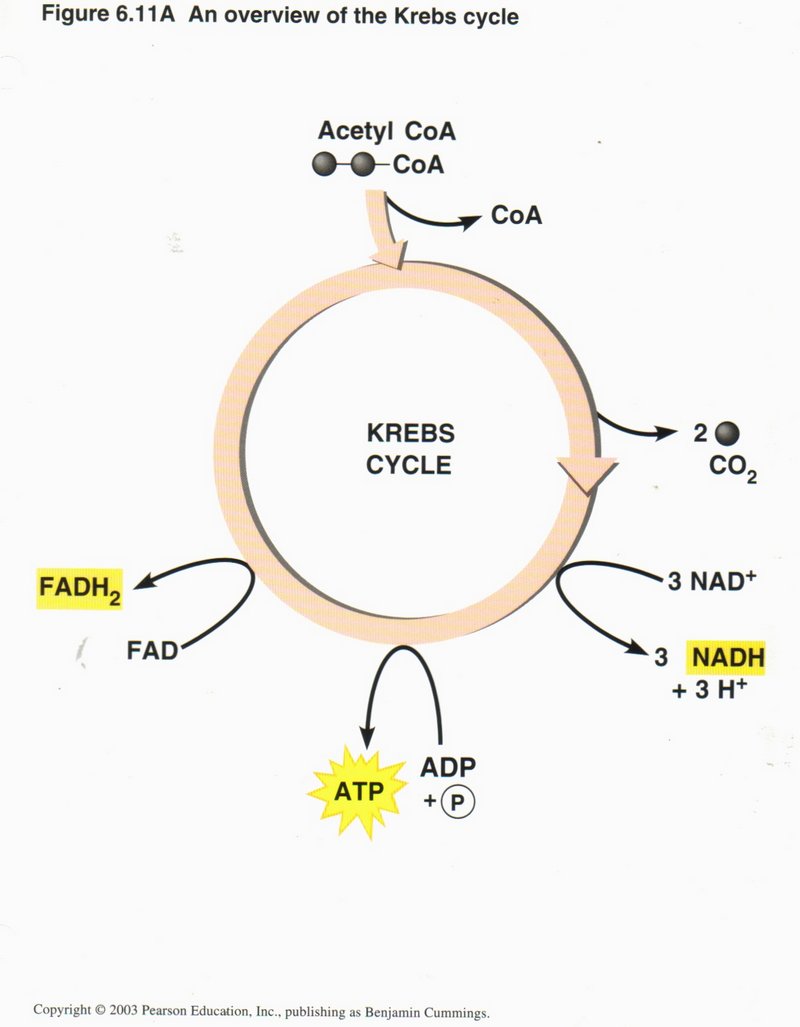
Here we see the overview of the Krebs cycle, where the net input is one molecule of acetyl CoA, three NAD+, one ADP plus
inorganic phosphorus, and one FAD. The net outputs are CoA, two CO2, three NADH plus three H+, one ATP, and one
FADH. The chemistry is quite complex and was first unraveled by Hans Krebs, hence the name of the cycle.
Click here for next page
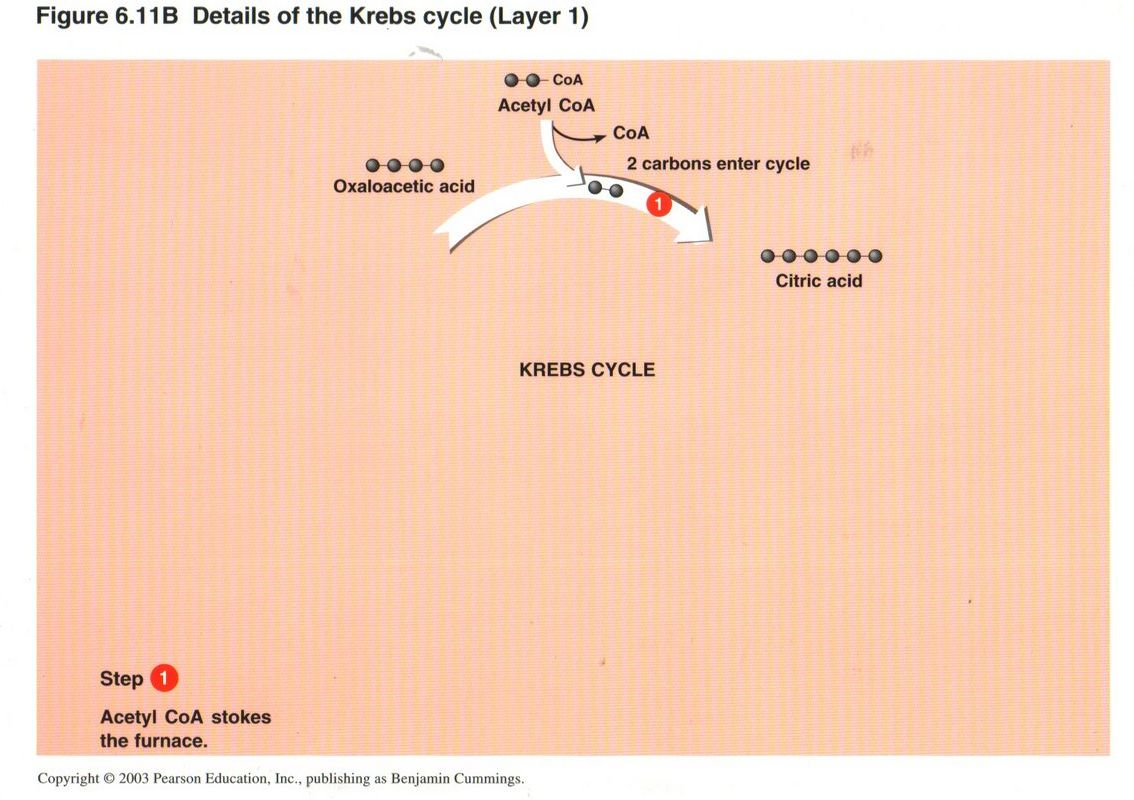
In the first step, CoA “stokes the furnace” by catalyzing the union of the two carbons of the acetyl radical with recycled 4-carbon
oxaloacetic acid to form 6-carbon citric acid. CoA then leaves the cycle to pick up another acetyl radical by the reduction of pyruvic
acid (or other source).
Click here for next page
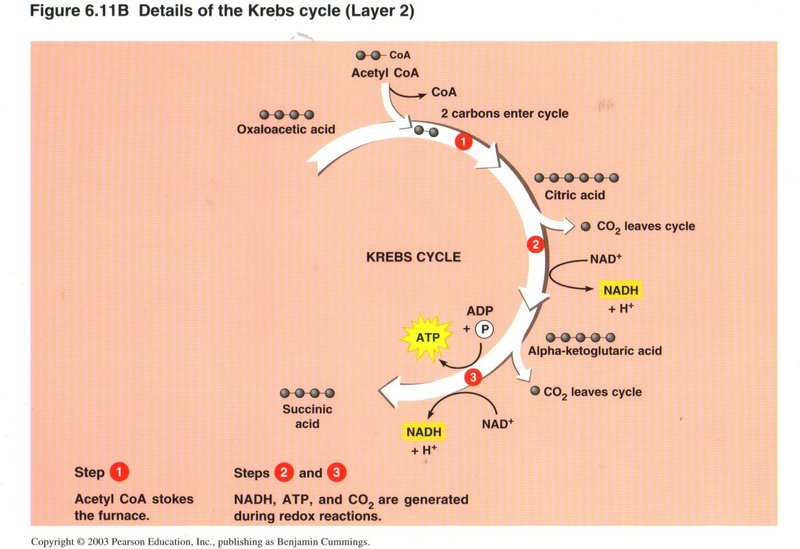
In step 2, the 6-carbon citric acid is reduced to 5-carbon alpha-ketoglutaric acid plus C02 with the upgrading of
NAD+ to NADH plus H+. In step 3, the 5-carbon molecule is reduced to 4-carbon succinic acid plus C02
with both the upgrading of another NAD+ to NADH plus H+, and ADP plus a phosphate radical to ATP.
Click here for next page
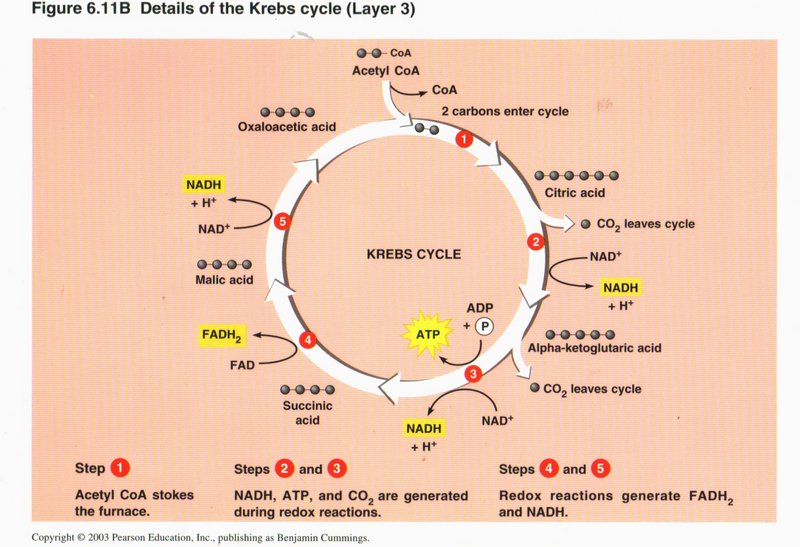
In step 4, the succinic acid is changed to malic acid, with the upgrading of FAD to FADH Finally, in step 5, the malic acid is converted
into oxaloacetic acid, with the upgrading of a third NAD+ to NADH plus H+, thus completing the cycle. The cycle
is also fed from other sources of acetyl-CoA in the cell. Remember that the Krebs cycle takes place in the mitochondrion, in which
chemiosmosis also takes place, as shown below.
Click here for next page
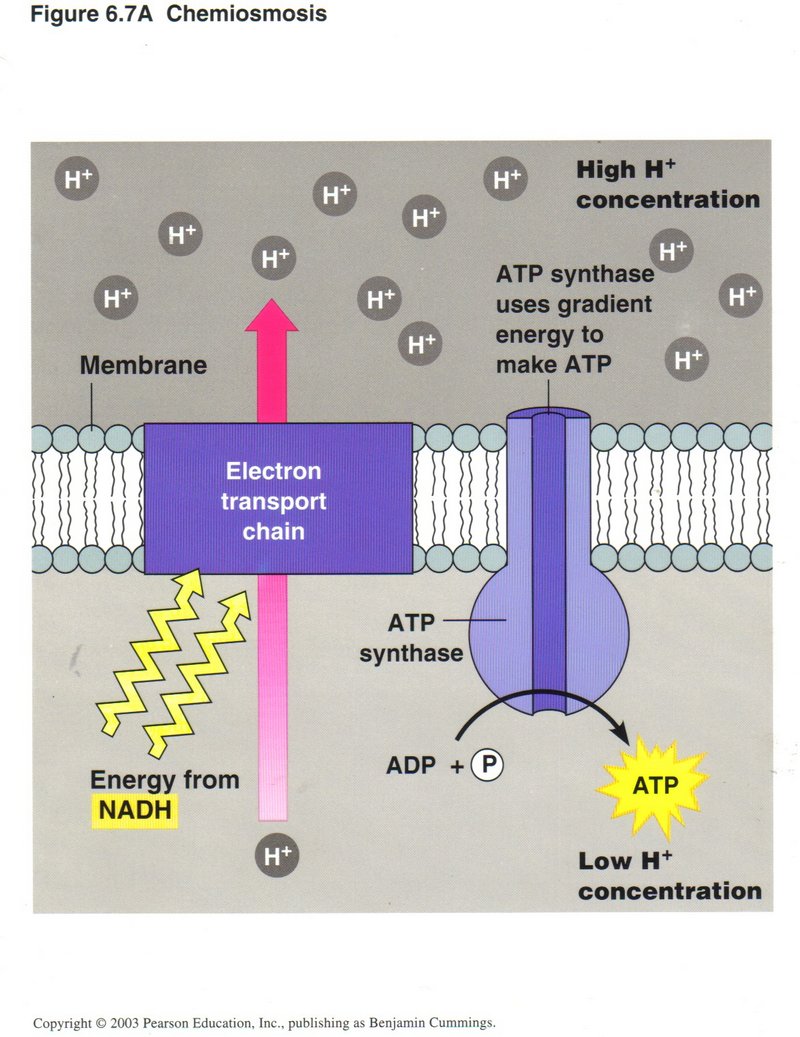
Chemiosmosis
In this view we see the use of one form of energy to generate another form of energy. The protein complex labeled “Electron transport
chain” in this view pumps H+ ions from the interior of the mitochondrion to its exterior, powered from NADH. The high
concentration of H+ thus produced outside of the membrane causes a flow of H+ ions through the protein complex
ATP synthase, providing the energy to upgrade ADP to ATP.
Click here for next page
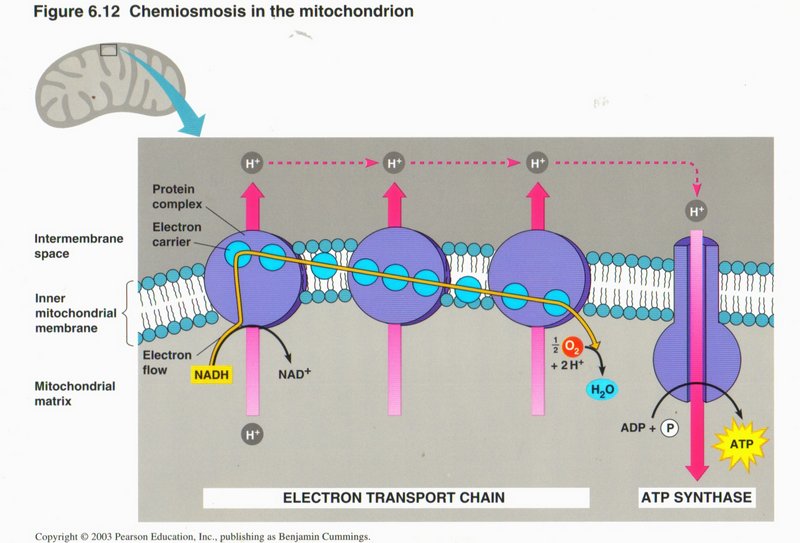
Here we see the electron tranport chain in more detail. The electrons stripped from the NADH are added to H+ ions in the
formation of a water molecule (H2O) from dissolved molecular oxygen (02).
Click here for next page

In this view, we see how poisons can block the normal machinery of the cell. Four of them block the pathways of electrons or H+
ions, and the fifth allows H+ ions to leak through the membrane and bypass the ATP synthase pump.
Click here for next page
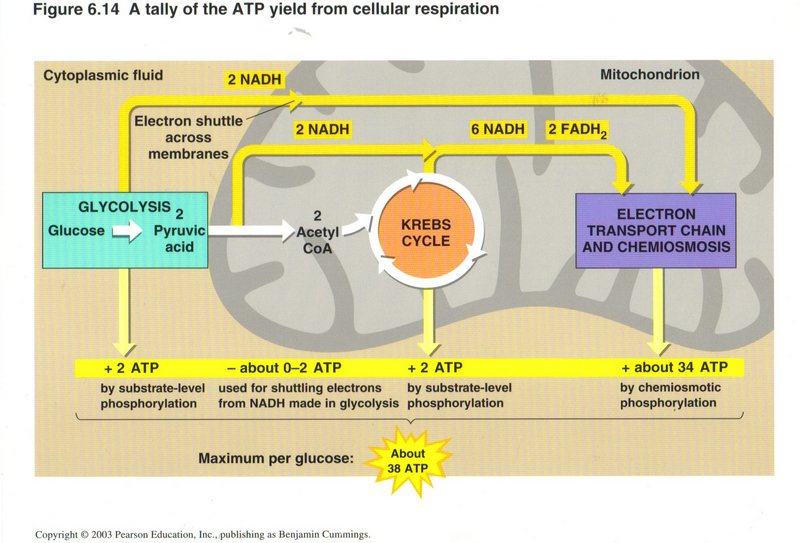
This view show total gain in energy of the three energy-producing processes. While the direct production of ATP from ADP by glycolysis
and the Krebs cycle is relatively small (2-4 molecules), the use of the NADH and FADH to power the ATP synthase pump in chemiosmosis
produces about 34 molecules of ATP from ADP. The maximum grand total is 38 molecules of ADP upgraded to ATP per molecule of glucose.
Click here for next page
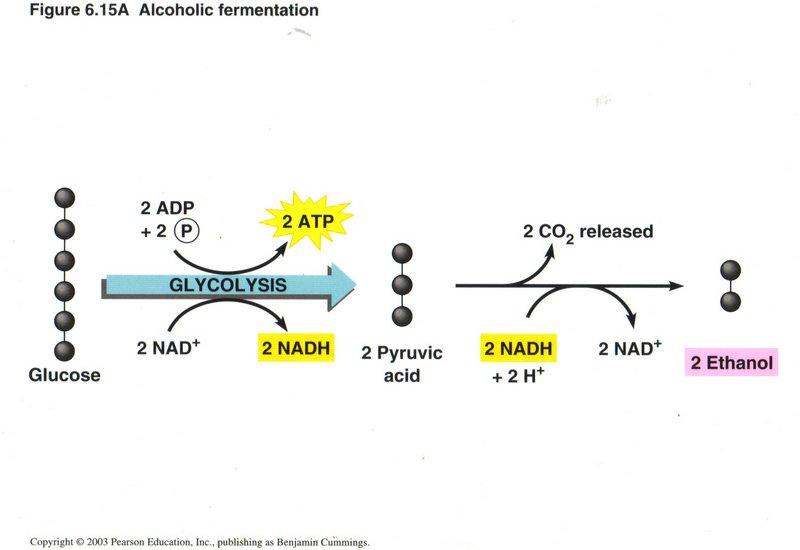
Fermentation
Some plant cells ferment the pyruvic acid into ethanol, the alcohol of intoxicating beverages, using the energy of the NADH produced
in the reduction of glucose to pyruvic acid, releasing two molecules of CO2
Click here for next page
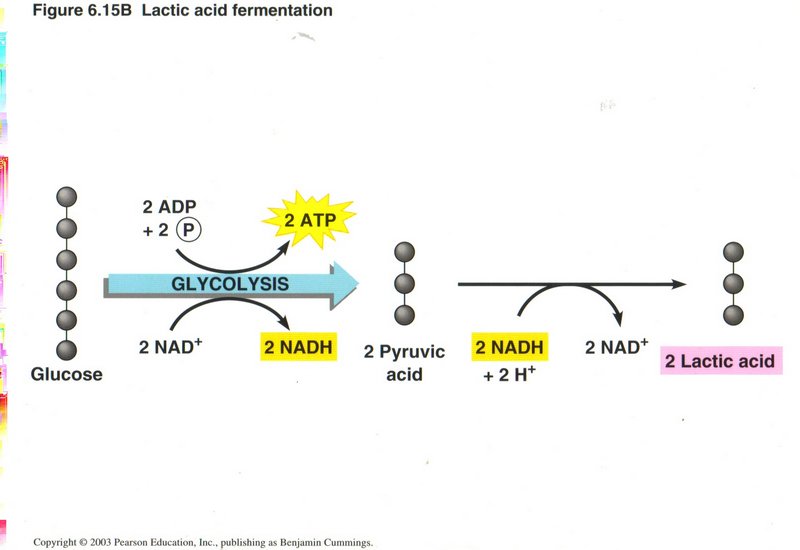
Aother example of fermentation from pyruvic acid, in which lactic acid is produced.
Click here for next page
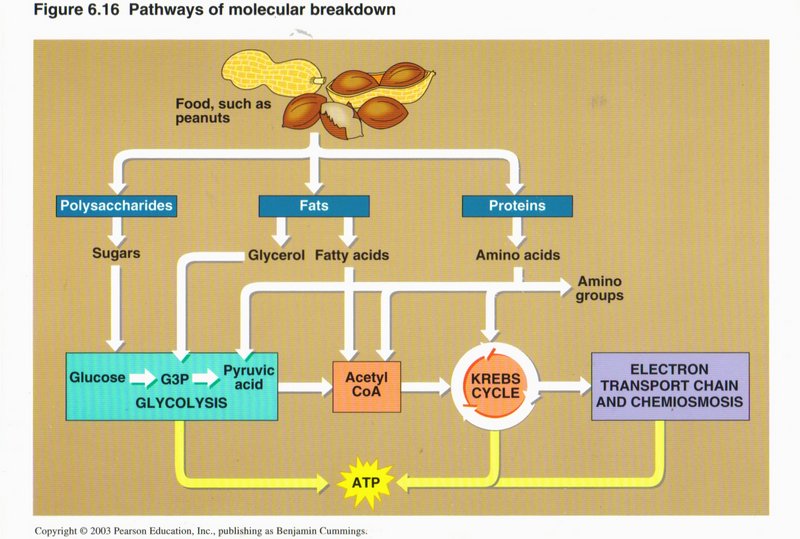
Breakdown and Synthesis of Foods
In this view we see how foods (such as peanuts in this example) are broken down by the cell to produce energy-source ATP. The complex
food molecules are first broken down into simpler substances (such as glucose and amino acids) by enzymes in the cytoplasm. The glucose
feeds glycolysis and the Krebs cycle, and the amino acids form the raw material for the proteins manufactured by the cell.
Click here for next page
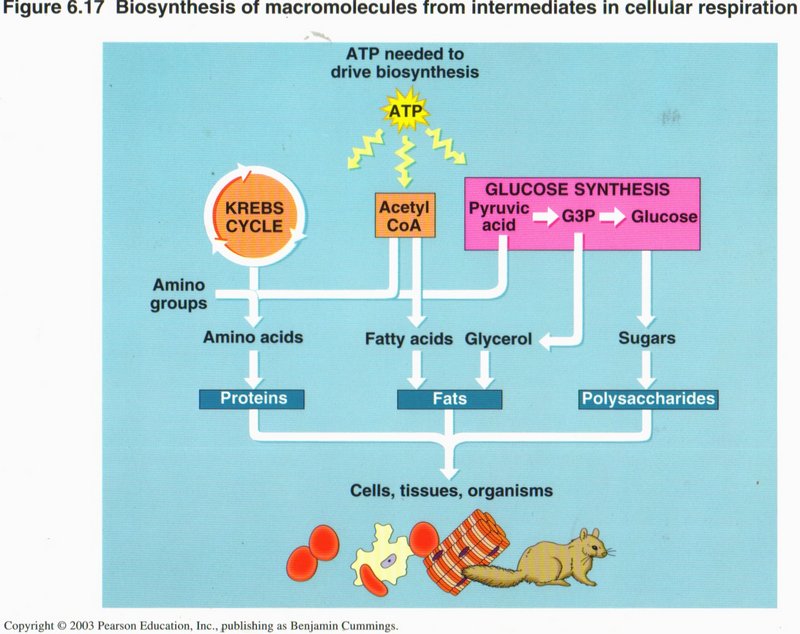
However, the proportions and types of different intermediate substances (such as amino acids) may not meet the full needs of the cell,
so it is able to manufacture the specific ones it needs, as illustrated in this chart.
Click here for next topic.
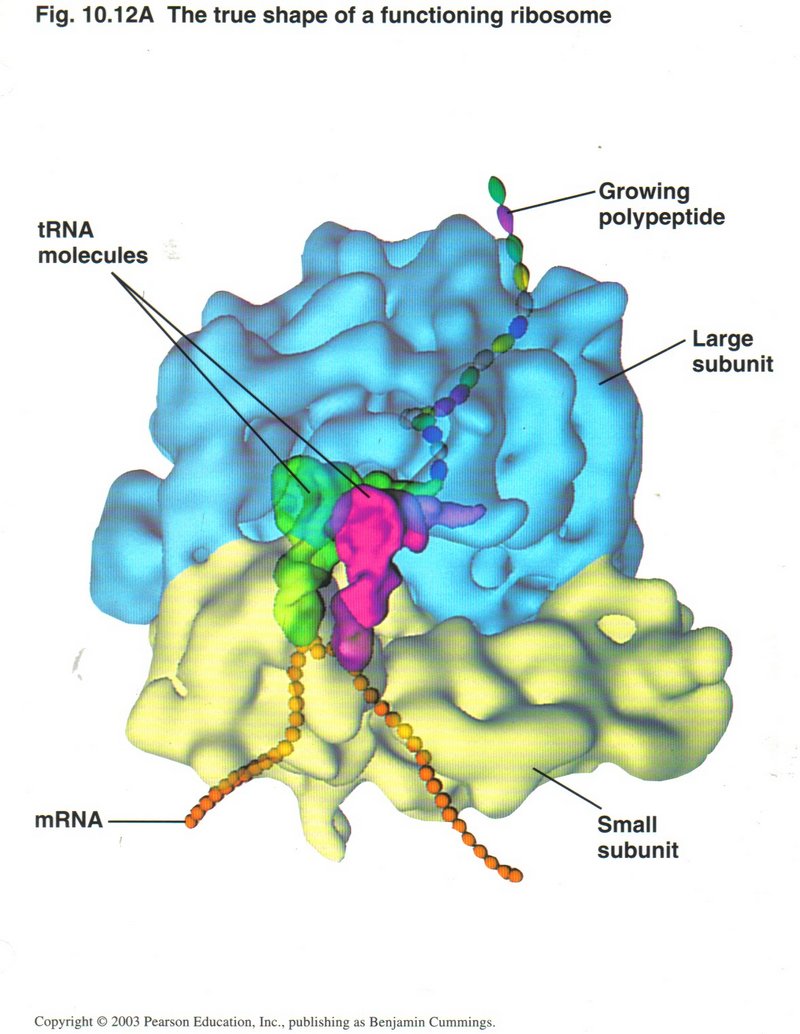

The entire process of transcription takes place in the nucleus of the cell. As it is generated, the mRNA ribbon passes through a pore
in the nuclear membrane to a ribosome attached to the rough ER, where translation will take place. Here we see the two main actors in
translation: On the left, an artist’s conception of what a ribosome looks like (left), and on the right a tRNA (transfer RNA) molecule.
The ribosome complex consists of the large ribosomal unit and the small ribosomal unit. It is formed from a
type of RNA called rRNA (ribosomal RNA) and several proteins. The tRNA, specific for each of the 20 types of amino acids, brings its
amino acid to the ribosome for incorporation into the growing polypeptide (as a proto-protein is called). This form of RNA is
shaped to bind at one end of the molecule with the particular amino acid it is associated with (see upper right) and bind to the mRNA
codon (three consecutive nucleotides) at the other end (see lower center). There are 20 different forms of tRNA ― one for each
of the 20 amino acids of which proteins are constituted.
Click here for next page
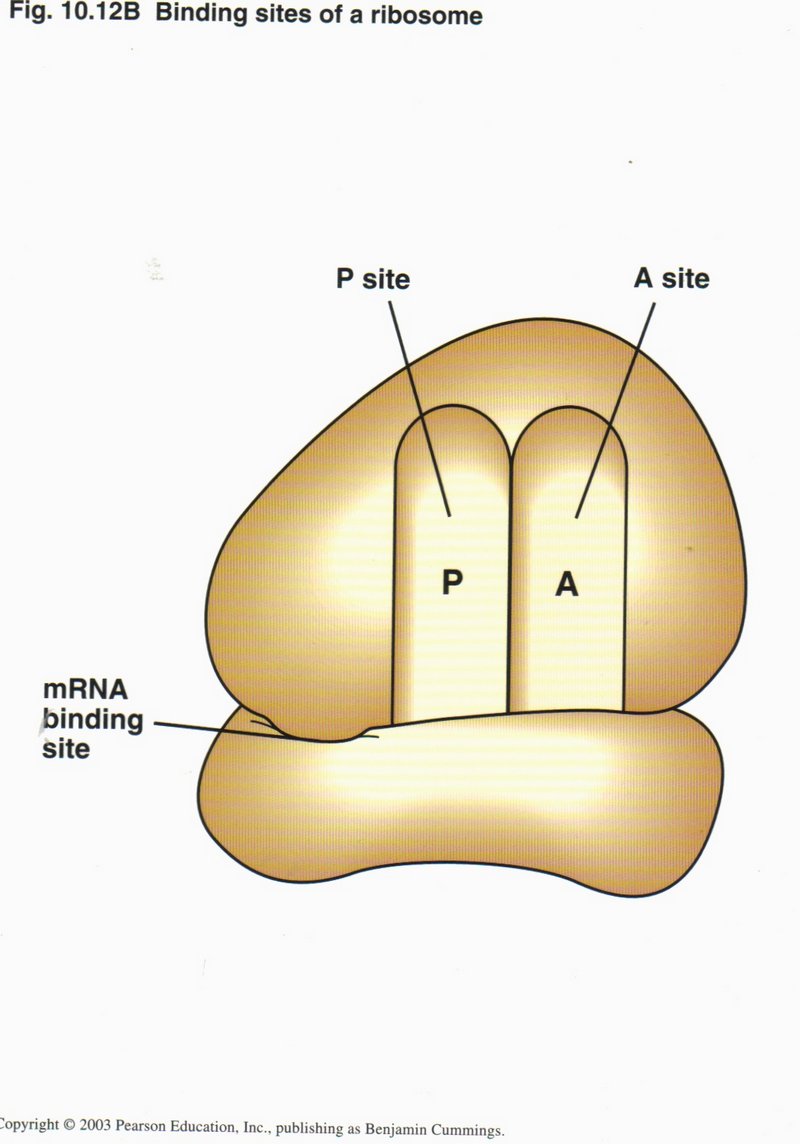
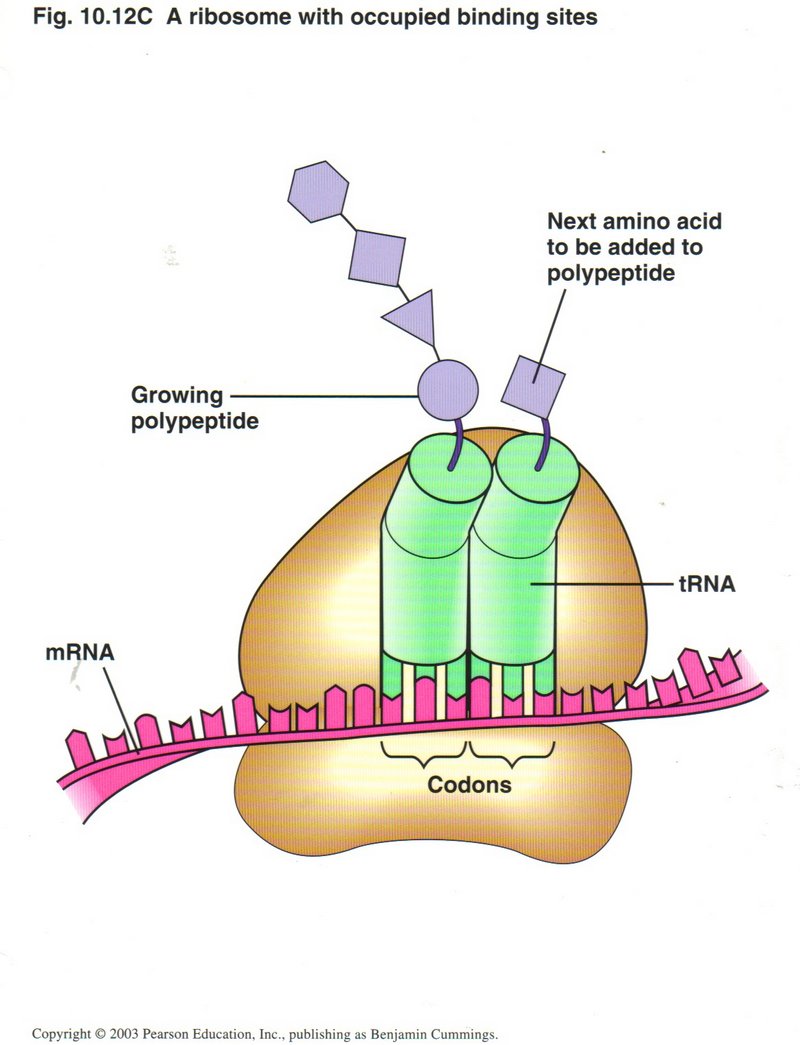 Translation is initiated by the binding of the small ribosonal subunit to the start codon AUG of the mRNA, together with the tRNA
for Met with its attached amino acid (left figure). The large ribosomal unit then binds to the complex so that the tRNA is located in
its P site (right figure). The large ribosomal unit acquires the next tRNA which matches the next codon of the mRNA, brings it into
the A site, and then connects its amino acid with the amino acid held in the P site. By a conformal change powered by ATP, the tRNA
in the P site is released, the mRNA is moved three nucleotides (one codon) to the left, shifting the tRNA from the A site to the
P site, and thus allowing a third tRNA to bring its amino acid to be connected into the growing polypeptide.
Translation is initiated by the binding of the small ribosonal subunit to the start codon AUG of the mRNA, together with the tRNA
for Met with its attached amino acid (left figure). The large ribosomal unit then binds to the complex so that the tRNA is located in
its P site (right figure). The large ribosomal unit acquires the next tRNA which matches the next codon of the mRNA, brings it into
the A site, and then connects its amino acid with the amino acid held in the P site. By a conformal change powered by ATP, the tRNA
in the P site is released, the mRNA is moved three nucleotides (one codon) to the left, shifting the tRNA from the A site to the
P site, and thus allowing a third tRNA to bring its amino acid to be connected into the growing polypeptide.
Click here for next page
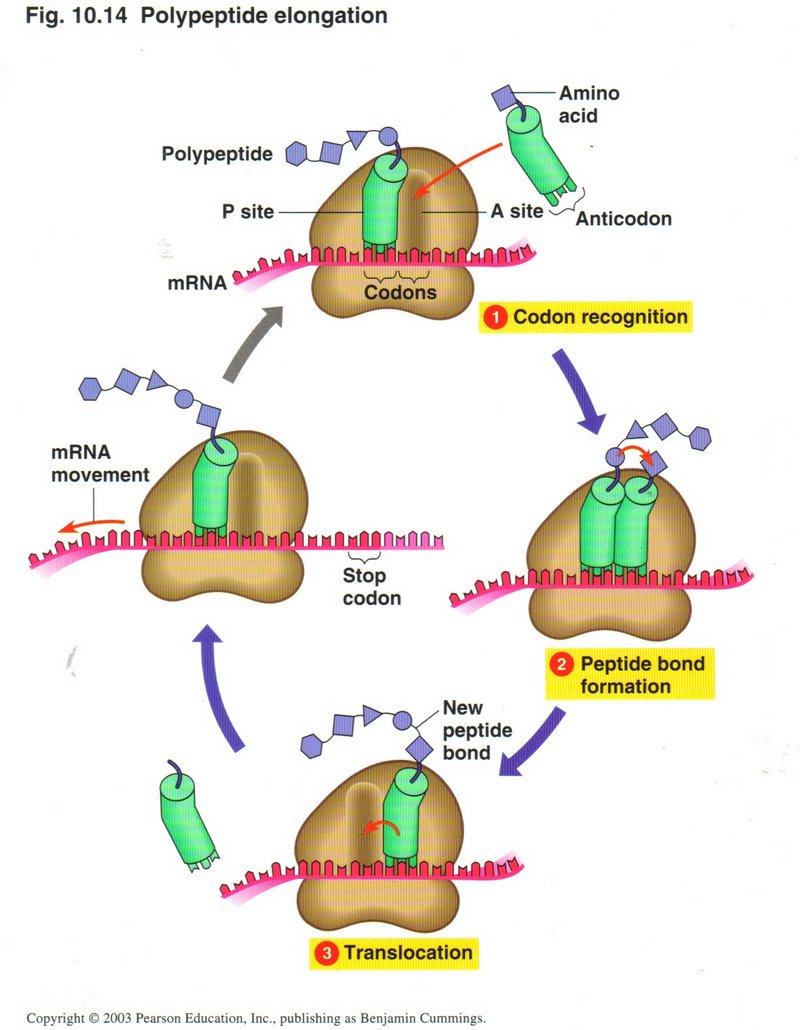
Here we see the whole process of elongation: (1) codon recognition, (2) peptide bond formation, (3) translocation, RNA movement.
These steps will continue until the large ribosomal unit recognizes one of the three stop codons.
Click here for next page
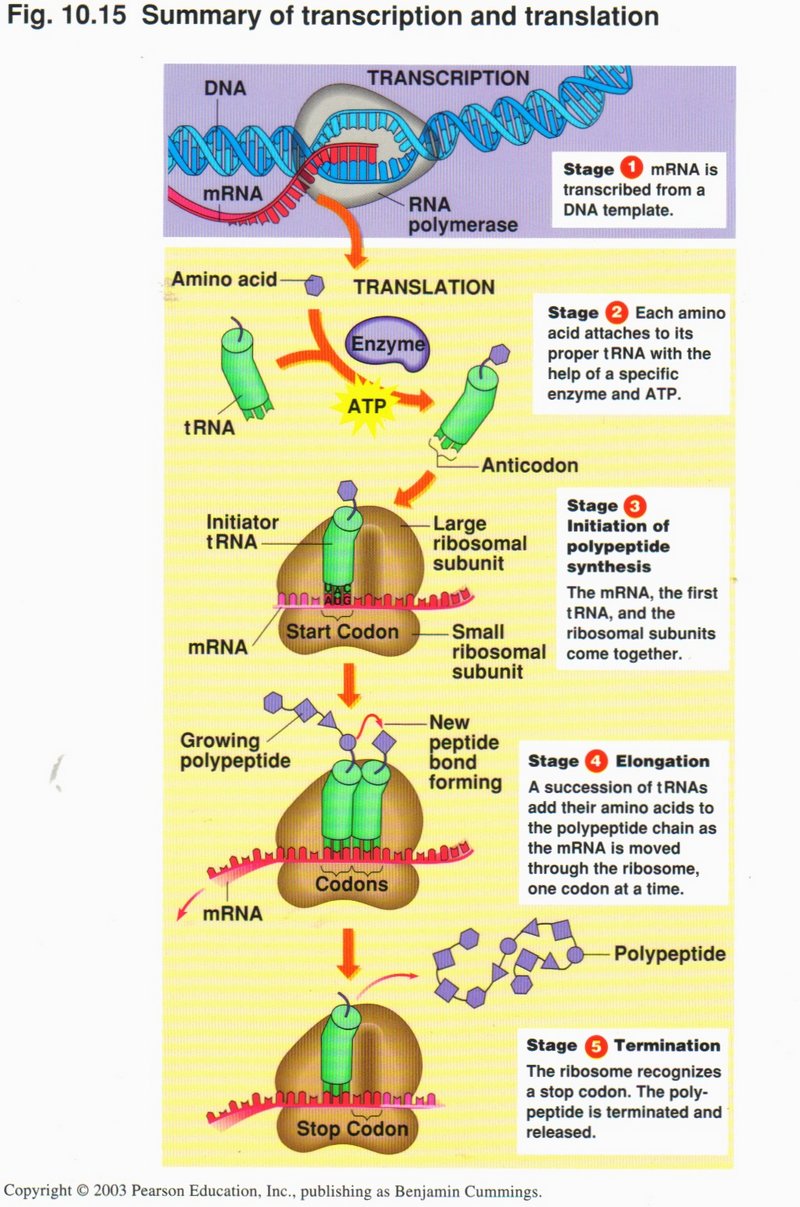
Summary of transcription and translation
Stage 1: mRNA is transcribed from a DNA template in the nucleus;
Stage 2: In the ribosome, each amino acid attaches to its proper tRNA with the help of a specific enzyme and ATP;
Stage 3: Initiation of polypeptide synthesis. The mRNA, the first tRNA, and the ribosomal subunits come together;
Stage 4: Elomgation. A succession of tRNAs add their amino acids to the polypeptide chain as the mRNA is moved through the ribosome,
one codon at a time;
Stage 5: Termination. The ribosome recognizes a stop code; the polypeptide is terminated and released.
Click here for next page
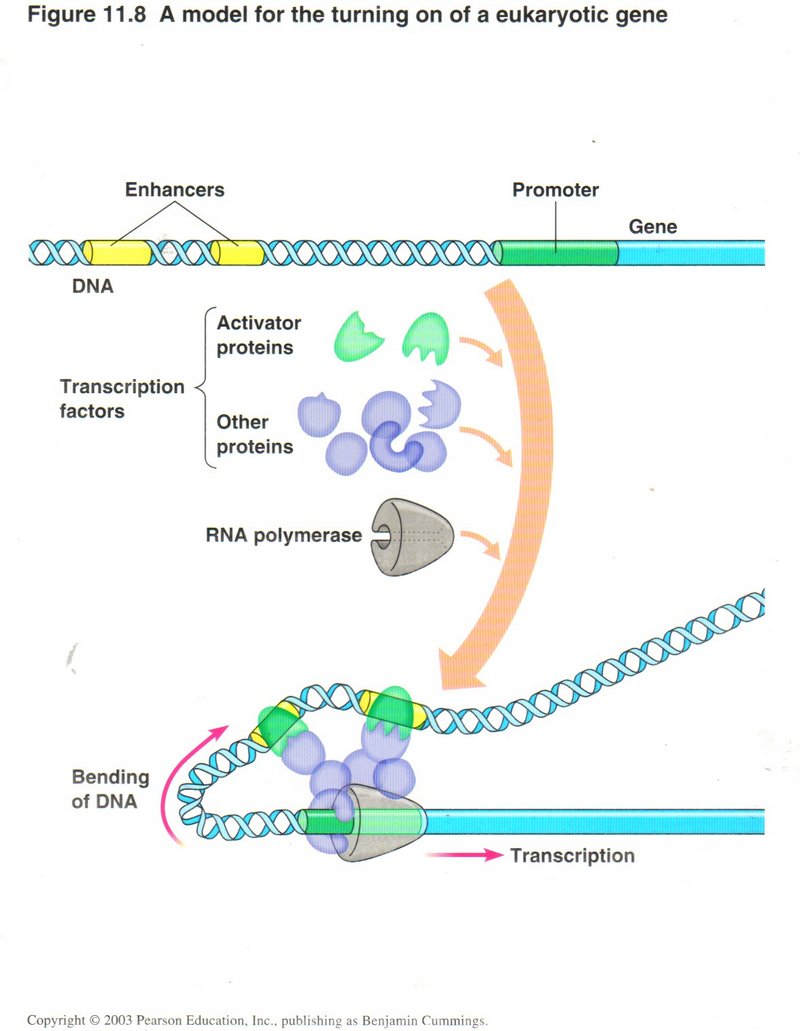
Regulation of gene expression
How does the cell know when to generate a particular protein (referred to as gene expression)? The DNA has two special sets of
nucleotides specific for each gene ― one or more enhancer sections and the promoter section. When the special proteins that recognize
these DNA sites (called transcription factors) are present, they, together with DNA polymerase, carry out the transcription of a gene.
They do so by binding to the enhancer and promoter special sections, as shown, thus causing the DNA helix to bend and bring the protein
complex together.
Click here for next page
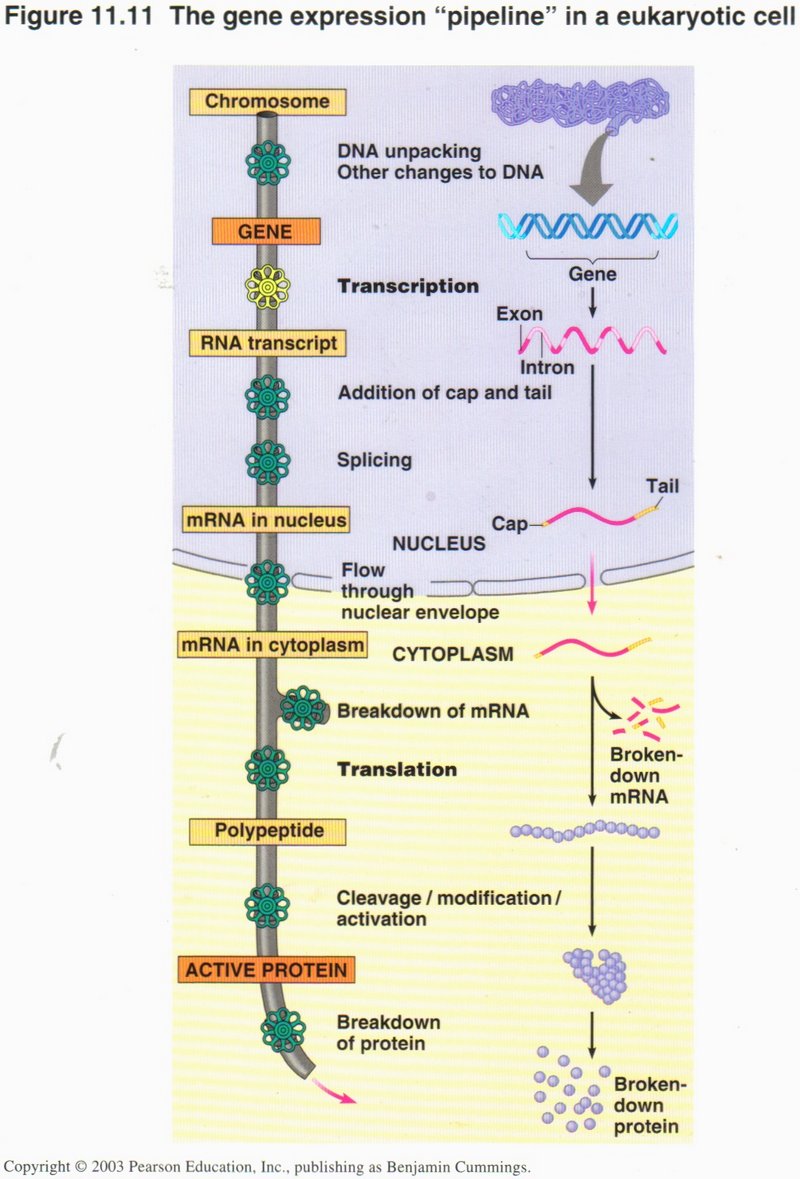
This chart shows the many places in the processes of protein generation where control is exercised (represented by a valve handle in
the pipeline). These controls are effected by activating or inhibiting the specific proteins needed for the function controlled. There
are literally thousands of proteins involved in these processes and their controls, most of which are present in the cytoplasm at all
times.
Click here for next page
Enzymes and How They Work
Using the analogy of jumping beans having to surmount a barrier to get out of their environment, we see that the enzyme (usually a
protein complex), in effect, reduces the barrier, so that reactant molecules of (much) less energy can take part in the reaction,
whereas without the enzyme, only the most energetic molecules of reactants could do so.
Click here for next page
Here we see how the enzyme works. The enzyme will have at least one binding site where a reactant (called a substrate) will fit
(bind) to the enzyme. Binding is usually caused by one or more hydrogen bonds (where the positive field of a hydrogen atom of one
(part of a) molecule will attract the negative field of a neighboring oxygen or nitrogen atom (in the same or different molecule).
(1) In the example, a molecule of sucrose approaches the enzyme at its binding site. (2) The sucrose binds to the enzyme. (3) The
enzyme adds a molecule of H to the sucrose molecule, causing it to divide into two molecules. (4) The enzyme changes its shape,
destroying the hydogen bonds and releasing the two products, in this case molecules of glucose and fructose.
Click here for next page
Here we see both the normal binding of a substrate and two ways another protein can inhibit the binding action, either by taking the
place of the substrate or by binding to another site on the enzyme, where its presence causes the enzyme to change shape and thus
prevent the binding of the substrate. Other proteins can function as activators of enzymes as well as inhibitors. In such cases the
normal shape of the enzyme will not fit the substrate. The activator protein binds to a second site, causing the enzyme to change
shape as to fit the substrate. Whenever an enzyme changes shape, it does so at the cost of a molecule of ATP.
Click here for next topic
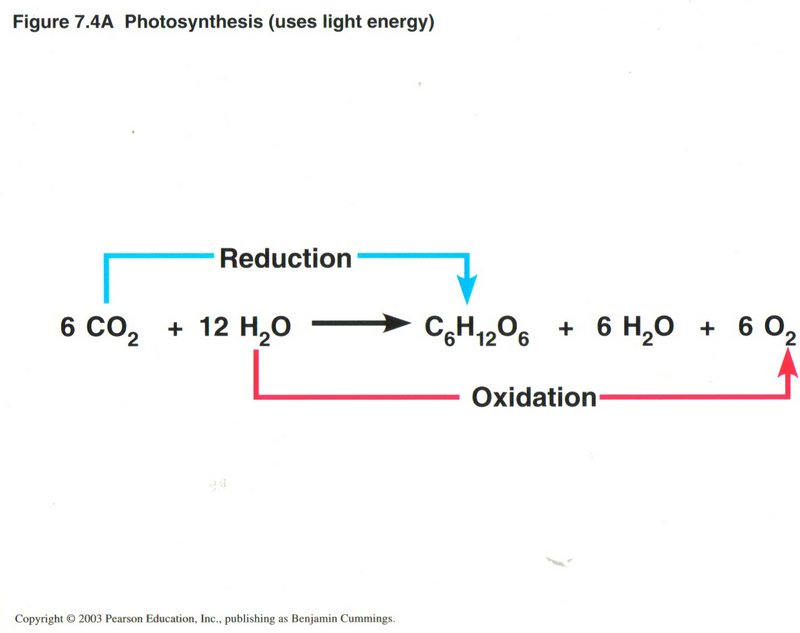
The O2 liberated by photosynthesis is made from the oxygen atoms in H2O (water). Like cellular respiration,
photosysnthesis is a redox (reduction/oxidation) process. Water molecules are split apart, and electrons and H+ ions are
removed (oxidation), leaving O2 gas. These electrons and and H+ are transferred to CO2 (reduction),
producing sugar. Unlike cellular respiration which releases energy, photosynthesis stores energy. The complete process of photosynthesis
consists of two linked sets of reactions: the light reactions and the Calvin cycle. The light reactions convert light energy to chemical
energy and produce O2. The Calvin cycle assembles sugar mlecules from CO2 using the energy-carrying products of
the light reactions.
Click here for next page
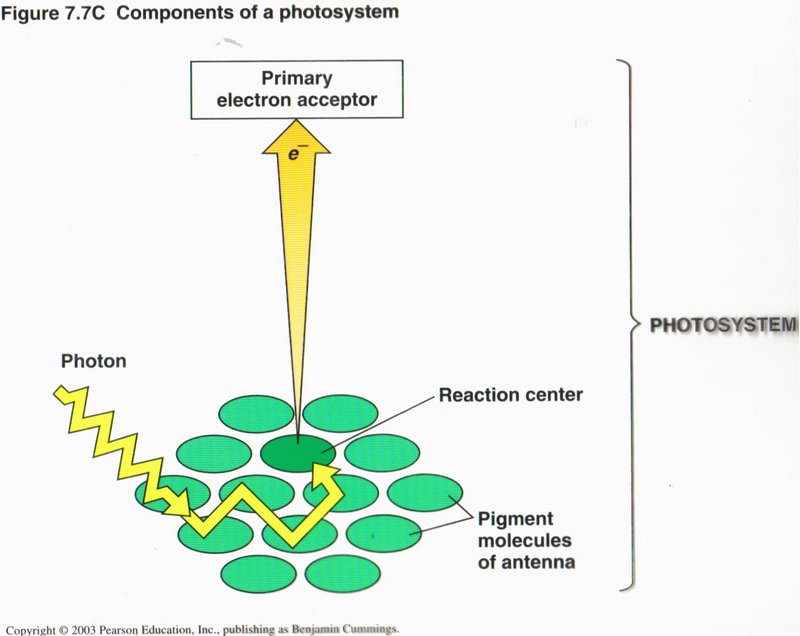
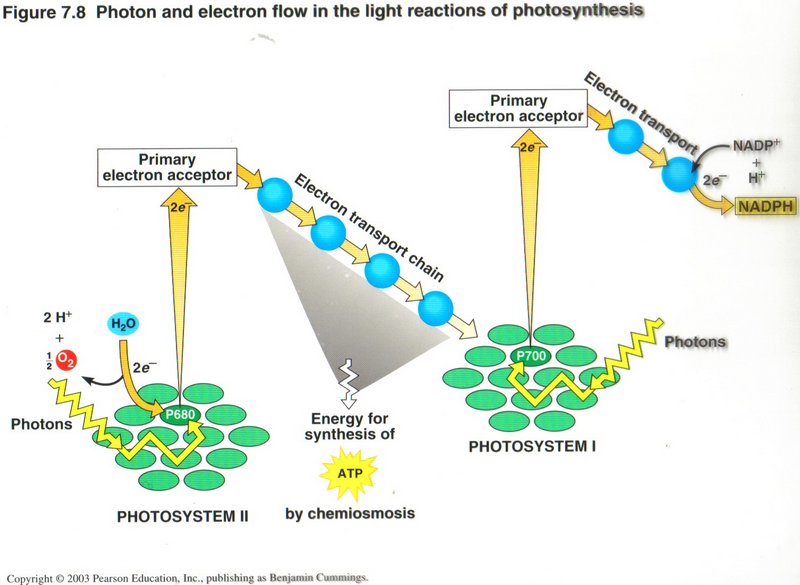
The Light Reactions: Converting Solar Energy to Chemical Energy. (See left diagram) Certain wavelengths of visible light
drive the light reactions of photosynthesis. Each of the many light-harvesting units of the chloroplast’s thylakoid membranes, called
photosystems, consists of an “antenna” of chlorophyll and other pigment molecules that absorb light and a primary electron receptor
that receives excited electrons from the reaction-center chlorophyll. (Now see right diagram) Two connected photosystems (I and
II) collect photons of light and transfer the energy to chlorophyll electrons, using slightly different frequencies of the incident
light. The excited electrons are passed from the primary electron acceptor to electron transport chains. Electrons shuttle from
photosystem II to photosystem I, and their energy ends up in ATP and NADPH. Photosystem II regains electrons by splitting water,
leaving O2 gas as a by-product.
Click here for next page
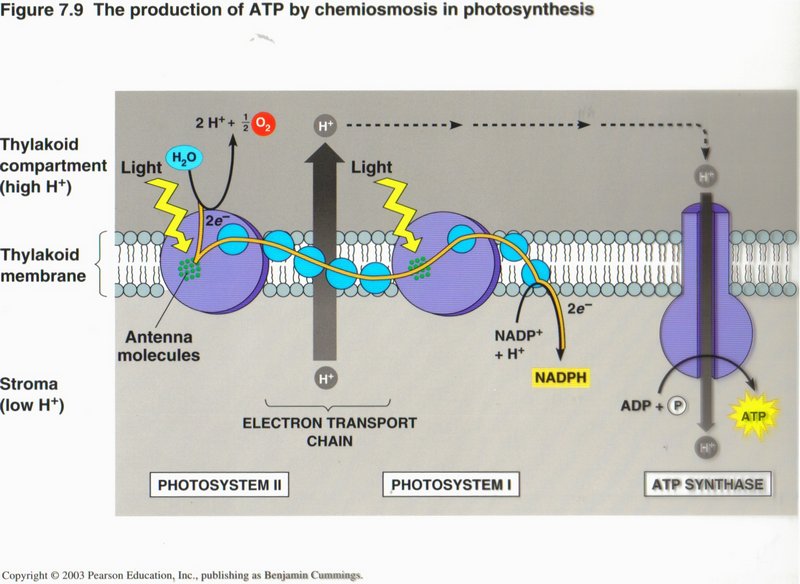
The ATP is synthesized by chemiosmosis. The electron transport chains are arranged with the photosystems in the thylakoid membranes
and pump H+ through that membrane. The flow of H+ back through the membrane is harnessed by ATP synthase to make
ATP (photophosphorylation). In the stroma, the H+ ions, electrons from the electron transport chain, and NADP+
combine to form NADPH.
Click here for next page
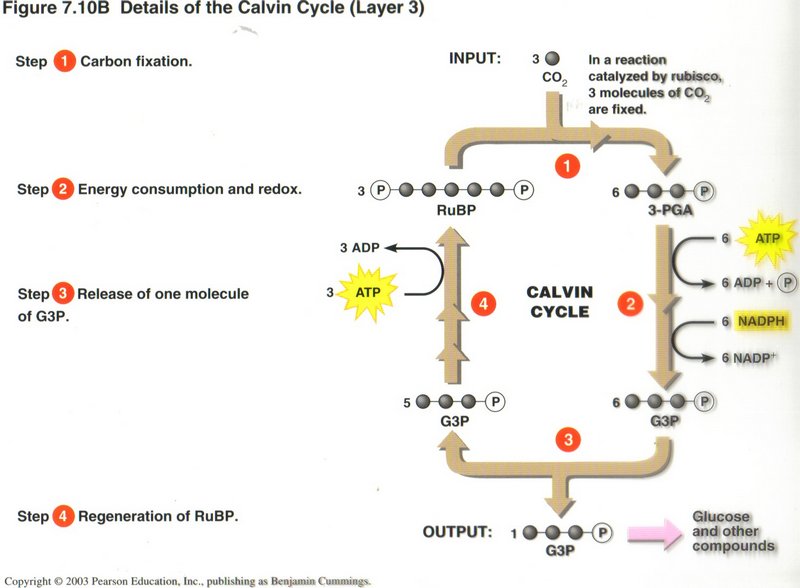
The Calvin Cycle: Converting CO2 to Sugars
Step 1: Carbon fixation. An enzyme called rubisco combines three molecules of CO2 with three molecules of a
five-carbon sugar called ribulose bisulphate (abbreviated RuBP). Six molecules of the three-carbon organic acid 3-phosphoglyceric
acid (3-PGA) result.
Step 2: Energy consumption and redox. Two chemical reactions (indicated by the two brown arrows) consume energy from six
molecules of ATP and oxidize six molecules of NADPH. Six molecules of 3-PGA are reduced, producing six molecules of the energy-rich
three-carbon G3P.
Click here for next page

Step 3: Release of one molecule of G3P. Five of the G3Ps remain in the cycle. The single molecule you see leaving the cycle
is the net production of photosynthesis. A plant cell uses two G3P molecules to make one molecule of glucose, which has six carbons.
Since the Calvin cycle incorporates only one molecule of CO2 — and thus only one carbon atom — at a time, it takes six
complete turns of the cycle to produce the two molecules of G3P that go into one glucose molecule.
Step 4: Regeneration of RuBP. A series of chemical reactions uses energy from ATP to rearrange the atoms in the five G3P
molecules, forming three RuBP molecules. These can start another turn of the cycle.
Click here for next page
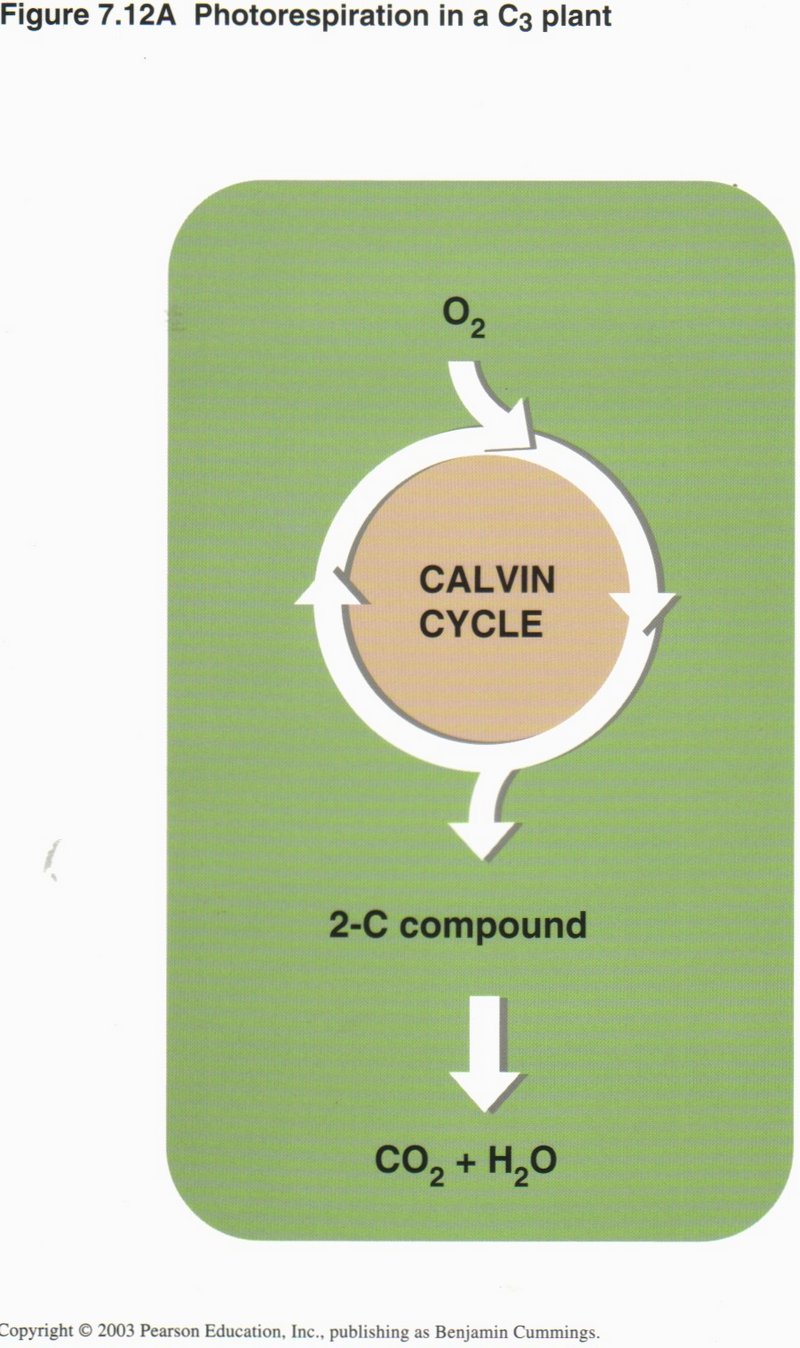
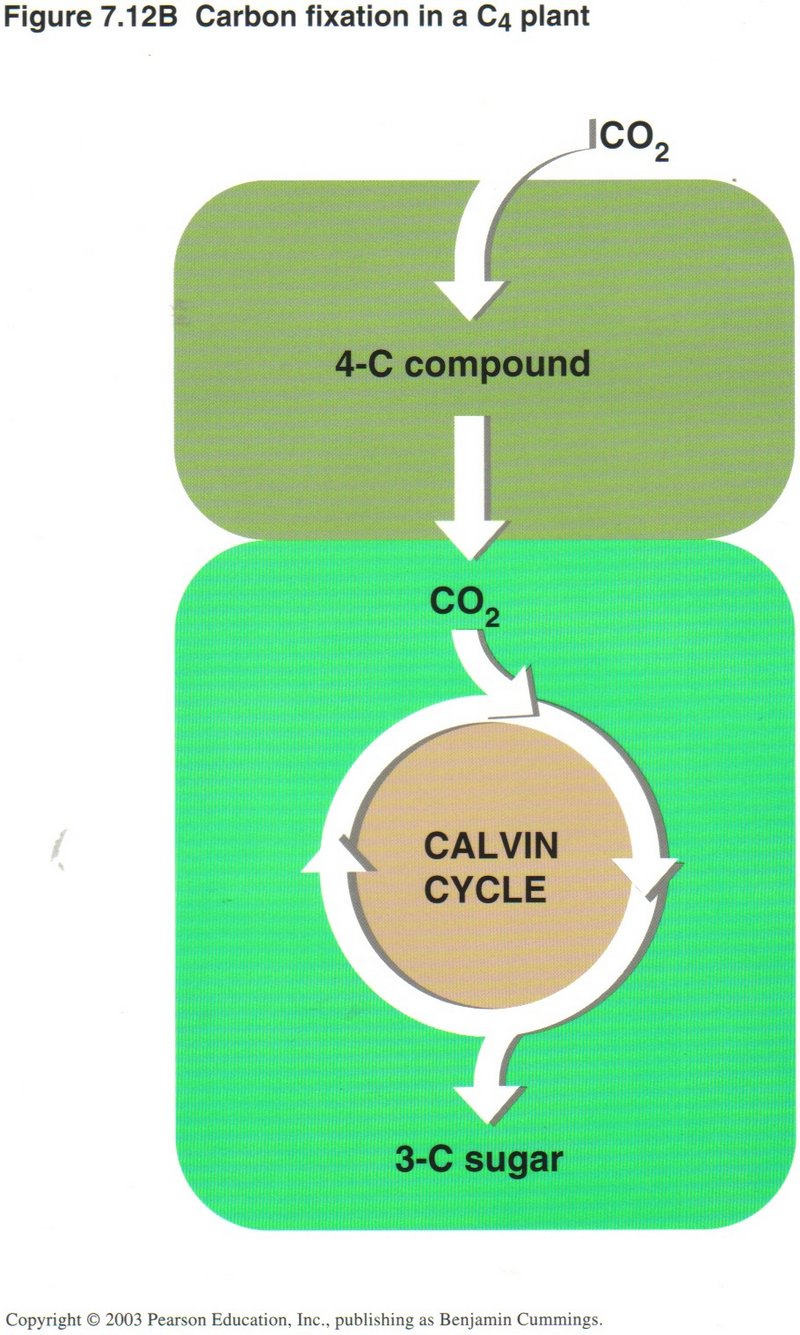
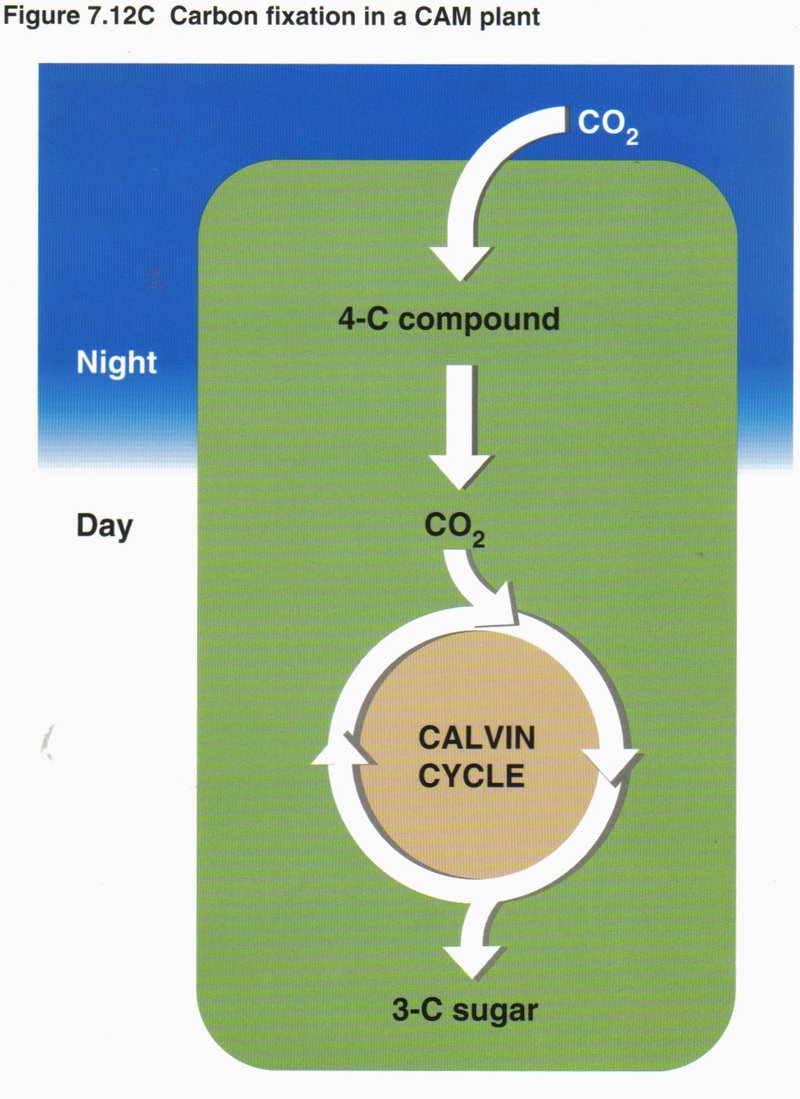
Photosynthesis Reviewed and Extended. Many plants make more sugar than they need; the excess is stored in roots, tubers, and
fruits, and is a major food source for animals. Photosynthesis produces billions of tons of organic matter each year and thus sustains
almost all life on Earth. Most plants are C3 plants , which take carbon directly from CO2 in the air and use it
in the Calvin cycle to build a three-carbon organic molecule. In such plants, stomata in the leaf surface usually close when the weather
is hot and dry. Although this mechanism saves water, it can cause a drop in CO2 and a buildup of O2 in the leaf,
diverting the Calvin cycle to an inefficient process called photorespiration (See left diagram). Some plants (See center
diagram) have special adaptations that enable them to save water and avoid photorespiration. Special cells in C4 plants,
such as corn and sugarcane, incorporate CO2 into a four-carbon compound that can donate CO2 to the Calvin cycle
in another kind of cell, compensating for the shortage of CO2 when the stomata are closed. Pineapples, many cacti, and most
succulents — the CAM plants (See right diagram) — employ a different mechanism. They open their stomata at night and make a
four-carbon compound that is used as a CO2 source by the same cell during the day.
Click here to for next topic.

 Here we see the structure of the eukaryotic (true nucleus) type of cell found in all life forms except bacteria; it is obviously
extremely complex. In this typical animal cell, the nucleus dominates the cell and contains the principal part of the DNA. It is in
the nucleus that the DNA is duplicated, a process called replication. It is
also in the nucleus that the generation of RNAs takes place in the process called transcription. There are several forms of
these. Messenger RNA (mRNA) is the ribbon from which proteins will be made in the ribosomes ― those little red dots
peppering the surfaces of the nuclear envelope and the rough ER (rough endoplasmic reticulum) ― in the process called
translation. Ribosomes are formed from another type of RNA ― rRNA (ribosonal RNA) ― plus several complex proteins. A
third type of RNA ― tRNA (transfer RNA) finds the specific amino acids needed to compose the protein and brings them to the
ribosome. The rough ER finishes the processing of the proto-protein (called a polypeptide) by chemically altering some of its
amino acid constituents and usually by folding it into its final shape. The rough ER also manufactures a number of chemical molecules
needed by the cell, like membrane units and enclosed vehicles, called vesicles, for carrying specific substances. The smooth
ER (smooth endoplasmic reticulum) manufactures fats and carbohydrates, including the sugars, needed to synthesize DNA and RNA.
Here we see the structure of the eukaryotic (true nucleus) type of cell found in all life forms except bacteria; it is obviously
extremely complex. In this typical animal cell, the nucleus dominates the cell and contains the principal part of the DNA. It is in
the nucleus that the DNA is duplicated, a process called replication. It is
also in the nucleus that the generation of RNAs takes place in the process called transcription. There are several forms of
these. Messenger RNA (mRNA) is the ribbon from which proteins will be made in the ribosomes ― those little red dots
peppering the surfaces of the nuclear envelope and the rough ER (rough endoplasmic reticulum) ― in the process called
translation. Ribosomes are formed from another type of RNA ― rRNA (ribosonal RNA) ― plus several complex proteins. A
third type of RNA ― tRNA (transfer RNA) finds the specific amino acids needed to compose the protein and brings them to the
ribosome. The rough ER finishes the processing of the proto-protein (called a polypeptide) by chemically altering some of its
amino acid constituents and usually by folding it into its final shape. The rough ER also manufactures a number of chemical molecules
needed by the cell, like membrane units and enclosed vehicles, called vesicles, for carrying specific substances. The smooth
ER (smooth endoplasmic reticulum) manufactures fats and carbohydrates, including the sugars, needed to synthesize DNA and RNA. The Golgi apparatus is the clearing house for cell traffic vesicles of all kinds. It also manufactures vesicles and
lysosomes, which are membrane enclosed vesicles containing potent enzymes capable of breaking down most complex organic
molecules into simpler substances used in manufacture in the smooth and rough ERs.
The Golgi apparatus is the clearing house for cell traffic vesicles of all kinds. It also manufactures vesicles and
lysosomes, which are membrane enclosed vesicles containing potent enzymes capable of breaking down most complex organic
molecules into simpler substances used in manufacture in the smooth and rough ERs. Here you can see something of the complexity of the plasma membrane surrounding the cell. Its main constituents are molecules of
phospholipids, which make up the double layer of the membrane. Since the fatty extensions (string-like molecules that point
to the center of the membrane) are water-repellant (like oil), the two layers of the membrane are continuously pressed together by
the watery media on the two sides (extra-cellular matrix on the outside and the cytoplasm on the inside). Studded throughout the
membrane are proteins of many kinds. Some provide recognition sites on the external side of the membrane for specific substances in
the extracellular medium. Some bind specialized messenger proteins (called hormones) that trigger many different functions in
the cell’s life cycle. Some provide pores which permit selected substances to enter or leave the cell. And some are involved with
moving ions (H+, Na+, Ca2+) (hydrogen, sodium, calcium positive ions) into or out of the cell.
Here you can see something of the complexity of the plasma membrane surrounding the cell. Its main constituents are molecules of
phospholipids, which make up the double layer of the membrane. Since the fatty extensions (string-like molecules that point
to the center of the membrane) are water-repellant (like oil), the two layers of the membrane are continuously pressed together by
the watery media on the two sides (extra-cellular matrix on the outside and the cytoplasm on the inside). Studded throughout the
membrane are proteins of many kinds. Some provide recognition sites on the external side of the membrane for specific substances in
the extracellular medium. Some bind specialized messenger proteins (called hormones) that trigger many different functions in
the cell’s life cycle. Some provide pores which permit selected substances to enter or leave the cell. And some are involved with
moving ions (H+, Na+, Ca2+) (hydrogen, sodium, calcium positive ions) into or out of the cell.
 An Overview of Photosynthesis. Photosynthesis is the process by which autotrophic (self-feeding) plants and some other organisms
use light energy to make sugar and oxygen gas from carbon dioxide and water. The following chemical equation shows the net input and
output of photosynthesis:
An Overview of Photosynthesis. Photosynthesis is the process by which autotrophic (self-feeding) plants and some other organisms
use light energy to make sugar and oxygen gas from carbon dioxide and water. The following chemical equation shows the net input and
output of photosynthesis:


































 The diagram illustrates meiosis II for a cell from a male organism; if the parental cell was from a female, cytokinesis I would have
placed most of the cytoplasm and organelles into one daughter cell and very little into the other. The phases of meiosis II are quite
similar to mitosis, the exception again being different cytokinesis for the granddaughter cells of the original female sex cell. The
daughter cell that got most of the cytoplasm divides with most of that cytoplasm and organelles going to one grand-daughter cell and
little to the other. The deprived daughter cell also divides, but the three depleted grand-daughter cells are subsequently destroyed,
leaving only the one cell which becomes an egg cell. In both the male and female types of meiosis, the grand-daughter (haploid)
cells are called gametes.
The diagram illustrates meiosis II for a cell from a male organism; if the parental cell was from a female, cytokinesis I would have
placed most of the cytoplasm and organelles into one daughter cell and very little into the other. The phases of meiosis II are quite
similar to mitosis, the exception again being different cytokinesis for the granddaughter cells of the original female sex cell. The
daughter cell that got most of the cytoplasm divides with most of that cytoplasm and organelles going to one grand-daughter cell and
little to the other. The deprived daughter cell also divides, but the three depleted grand-daughter cells are subsequently destroyed,
leaving only the one cell which becomes an egg cell. In both the male and female types of meiosis, the grand-daughter (haploid)
cells are called gametes.



































 Some membranes in the cell are permeable (one the molecules can transit). Molecules in solution migrate throughout the domain
available to them. If a higher concentration exists on one side of a permeable membrane, they will quickly move through the membrane
until the concentrations on the two sides are equal. No energy is needed.
Some membranes in the cell are permeable (one the molecules can transit). Molecules in solution migrate throughout the domain
available to them. If a higher concentration exists on one side of a permeable membrane, they will quickly move through the membrane
until the concentrations on the two sides are equal. No energy is needed.




 The view at the left shows the structure of the RNA nucleotide (uracil), and that on the right the structure of the DNA nucleotide
thymine. The only two variations are the presence of an OH group on the 2' carbon atom, making the sugar "ribose" in RNA rather than
the sugar "deoxyribose" in DNA (hence the first letter in the acronym DNA or RNA); and the substitution of uracil (U), which has a
single H atom on one of the carbon atoms in the ring rather than H3C on that atom in thymine. All the other bases in RNA
are the same as in DNA. However, these apparently minor differences make a major difference in the behavior of the two molecules.
Whereas RNA is stable in fairly long single strings of base-pairs, DNA is unstable in its single-string form for even short strings.
On the other hand RNA is not stable in a double helix form as is DNA (although some viruses make a short hybrid helix, one-half RNA,
the other half DNA), nor can it attain the enormous lengths that DNA usually does.
The view at the left shows the structure of the RNA nucleotide (uracil), and that on the right the structure of the DNA nucleotide
thymine. The only two variations are the presence of an OH group on the 2' carbon atom, making the sugar "ribose" in RNA rather than
the sugar "deoxyribose" in DNA (hence the first letter in the acronym DNA or RNA); and the substitution of uracil (U), which has a
single H atom on one of the carbon atoms in the ring rather than H3C on that atom in thymine. All the other bases in RNA
are the same as in DNA. However, these apparently minor differences make a major difference in the behavior of the two molecules.
Whereas RNA is stable in fairly long single strings of base-pairs, DNA is unstable in its single-string form for even short strings.
On the other hand RNA is not stable in a double helix form as is DNA (although some viruses make a short hybrid helix, one-half RNA,
the other half DNA), nor can it attain the enormous lengths that DNA usually does.






















 Translation is initiated by the binding of the small ribosonal subunit to the start codon AUG of the mRNA, together with the tRNA
for Met with its attached amino acid (left figure). The large ribosomal unit then binds to the complex so that the tRNA is located in
its P site (right figure). The large ribosomal unit acquires the next tRNA which matches the next codon of the mRNA, brings it into
the A site, and then connects its amino acid with the amino acid held in the P site. By a conformal change powered by ATP, the tRNA
in the P site is released, the mRNA is moved three nucleotides (one codon) to the left, shifting the tRNA from the A site to the
P site, and thus allowing a third tRNA to bring its amino acid to be connected into the growing polypeptide.
Translation is initiated by the binding of the small ribosonal subunit to the start codon AUG of the mRNA, together with the tRNA
for Met with its attached amino acid (left figure). The large ribosomal unit then binds to the complex so that the tRNA is located in
its P site (right figure). The large ribosomal unit acquires the next tRNA which matches the next codon of the mRNA, brings it into
the A site, and then connects its amino acid with the amino acid held in the P site. By a conformal change powered by ATP, the tRNA
in the P site is released, the mRNA is moved three nucleotides (one codon) to the left, shifting the tRNA from the A site to the
P site, and thus allowing a third tRNA to bring its amino acid to be connected into the growing polypeptide.














